TITRATION CURVES
advertisement

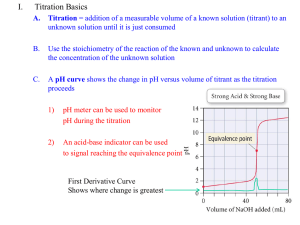
TITRATION CURVES The way the pH changes during an acid-base titration depends on the relative strengths of the acid and base. Four characteristic graphs can be produced for the various combinations of strong and weak acids and bases. A] Strong acid-strong base. 50.0 mL of 0.100 mol L-1 HCl with 0.100 mol L-1 NaOH B] Weak acid-strong base 50.0 mL 0.100 mol L-1 ethanoic acid with 0.100 mol L-1 NaOH LRY 2013 C] Strong acid-weak base 50.0 mL 0f 0.100 mol –1 ammonia with 0.100 mol L-1 HCl D] Weak acid-weak base 50.0 mL of 0.100 mol L-1 ethanoic acid with 0.100 mol L-1 ammonia solution. Equivalence point: The equivalence point is the point at which the amount of acid and base are equal. LRY 2013 Close to the equivalence point a small addition from the burette will cause a large change in pH. You can read the equivalence point of the graph by finding the centre of the vertical (steepest slope) section. For strong acid-strong base titration the equivalence point is at a pH of 7, indicating that [H+] = [OH-]. For weak acid-strong base titration the equivalence point is about a pH of 9, indicating that [H+] < [OH-]. The excess OH- is produced by hydrolysis of the conjugate base of the weak acid i.e. CH3COO-. The reverse is true for strong acid-weak base titration. The equivalence point is acidic due to the hydrolysis of the conjugate acid of the weak base (NH4+). In the case of weak acid-weak base the equivalence point is approx. pH 7 but it is not clearly defined. This titration is avoided whenever possible. Buffer zone: Buffers are formed when we mix weak acids (or bases) with their conjugate bases (or acids). Whenever a weak acid (or base) is involved in a titration we are going to make a buffer. The buffer zone on the pH graphs is centred halfway to the equivalence point, i.e. the point at which the concentration of the acid (or base) and its salt are equal. At that point the pH of the solution is equal to the pKa of the acid. Indicators: Indicators are weak acids whose conjugate bases are a different colour from their acids. The colour change occurs when [acid] = [base]. When the pH of the solution < pKa of the indicator, the indicator is one colour. When the pH > than pKa of the indicator, the indicator is a second colour. The colour change generally occurs over 2 pH units. When the indicator changes colour pH = pKa of the indicator. The correct indicator for a titration can be chosen by getting one whose pKa is in the region of the steepest part of the titration curve. Indicator Methyl red Methyl orange Phenolphthalein Bromothymol blue Cresyl blue LRY 2013 pKa value 5 4 9 7 11 pH range 4–6 3–5 8 – 10 6–8 10 -12 Example: Use the graph below to answer the following questions. The titration is for 25 mL 0.1 mol L-1 ammonia with dilute HCl Titration HCl versus ammonia 14 13 12 11 10 9 pH 8 7 6 5 4 3 2 1 0 0 5 10 15 20 25 30 35 40 45 50 Volume HCl a. b. c. d. e. LRY 2013 What is the pH at the beginning of the titration? What volume of acid has been added at the equivalence point? Label the buffer zone. At what point in the titration is the pH of the solution equal to the pKa? Reading from the curve, what is the magnitude of pka(NH4+)?