LAB- Catalase
advertisement

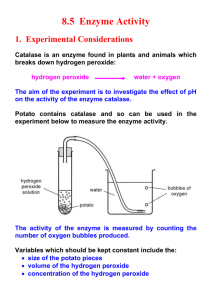
BIOLOGY LAB: CATALASE Observing an Enzyme INTRODUCTION: What would happen to your cells if they made a poisonous chemical? You might think that they would die. In fact, your cells are always making poisonous chemicals. They do not die because your cells use enzymes to break down these poisonous chemicals into harmless substances. Enzymes are proteins that speed up the rate of reactions that would otherwise happen more slowly. The enzyme is not altered by the reaction. You have hundreds of different enzymes in each of your cells. Each of these enzymes is responsible for one particular reaction that occurs in the cell. In this lab, you will study an enzyme that is found in the cells of many living tissues. The name of the enzyme is catalase (KAT-uh-LAYSS); it speeds up a reaction which breaks down hydrogen peroxide, a toxic chemical, into 2 harmless substances--water and oxygen. The reaction is as follows: This reaction is important to cells because hydrogen peroxide (H2O2) is produced as a byproduct of many normal cellular reactions. If the cells did not break down the hydrogen peroxide, toxic levels would accumulate and destroy the cell. In this lab, you will study the catalase found in beef or chicken liver cells. Even though catalase is an enzyme found in many cells, it is found in high levels in liver cells because the liver often functions to break down toxins present in the blood. It might seem strange to use dead cells to study the function of enzymes. This is possible because when a cell dies, the enzymes remain intact and active for several weeks, as long as the tissue is kept refrigerated. PRELAB REVIEW: Recall that the substrate is the molecule that the enzyme acts on, the products are the molecules produced by the reaction and enzymes are “reusable.” Under certain conditions enzymes are denatured. An enzyme is denatured when the protein molecule loses its proper 3-dimentional shape and can no longer function. Some things that can denature an enzyme are high temperatures, extremes of pH, heavy metals, and alcohol. MATERIALS: *8 Test tubes *10-ml Graduated cylinder *Fresh liver, chicken meat, and Potato *3% Hydrogen peroxide solution *Distilled water *Stirring rod *Test Tube rack *Tweezers *Waste cup *1 Beaker (to hold test tubes) *Petri dish FOR THE CLASS: *Thermometers *Ice water bath (0ºC) *water bath #1 (36ºC) *water bath #2 (80ºC) *Boiling water bath (100ºC) PROCEDURE: **You are responsible for cleaning your group’s lab supplies, lab table, and sink. Liquids may be poured down the sink, but solids (ex: potato cells, apple cells, paper towels) must be thrown away in the trash!!!** Part I Setting up the control. NOTE: Be sure to clean your stirring rod (and test tubes) between steps. 1. Place 1 dropper full of liquid liver into a clean test tube. 2. Place 2 ml of the 3% hydrogen peroxide solution into a graduated cylinder. 3. Add the hydrogen peroxide to the test tube and carefully observe the rate of reaction (how rapidly the solution bubbles/ its speed). 4. Throughout this investigation you will estimate the rate of the reaction (how rapidly the solution bubbles) on a scale of 0-5 (0=no reaction/no bubbles, 1=slow,...., 5= very fast). Assume that the reaction in step 3 proceeded at a rate of "4." 5. Recall that a reaction that absorbs heat is endothermic; a reaction that gives off heat is exothermic. Now, feel the temperature of the test tube with your hand. Take note of this information for your Data Table. Is the Catalase reusable? 6. Use a stir rod to break up the bubbles in your test tube. 7. Add another 2 mL of 3% hydrogen peroxide. Do you see oxygen bubbles forming again? Take note of this information so you can record it in the “Observation” section of your data table under “Room Temperature” liver. 8. Rinse and clean out this test tube and return it to your station. 9. Go back to your seat. SET UP DATA TABLES In order to set up your data tables, you need to know that we will be conducting two different experiments: 1) Effect of Temperature on the Rate of reaction in Liver Cells and 2) The Effect of Cell type on the Rate of Catalase Reaction. Read the lab directions for Temperature (Part 2). What is the IDV and what is the DV? Which goes in the first column? Write a descriptive title for the table which includes both the DV and IDV variable and then set up your table. Be sure to include a column for “Observations.” Now do the same thing for Types of cells (Part 3). Now that you have your data tables, you can add your rate of reaction data for liver cells at room temperature to both data tables. Under your Temperature data table, this is the room temperature (22ºC) data. Be sure to include your observations regarding whether enzymes are reusable & if the reaction was exothermic or endothermic under “Observations.” You can also use this same rate of reaction data for your cell type table. Part 2 Setting up the temperature tests 1. Add 1 dropper of liquid liver to three more clean test tubes (1 dropper amount per test tube). 2. Place 2 ml of the 3% hydrogen peroxide solution into each of three more clean test tubes (2ml per test tube). 3. Place one test tube of hydrogen peroxide in the ice water bath (there is a test tube with liver already in the freezer getting cold). 4. Place one test tube of liver and one test tube of hydrogen peroxide in warm water bath #1 (37ºC). 5. Place one test tube of liver and one test tube of hydrogen peroxide in warm water bath #2 (70ºC). 6. Put the final liver test tube in a boiling water bath. 7. Leave each test tube in the appropriate temperature for 15 minutes. 8. Conduct Part 3 of the experiment while you are waiting. Part 3 Is Catalase found in other types of cells? You will now test for the presence of Catalase in tissues other than liver. 1. Place 2 ml of the 3% hydrogen peroxide solution into the graduated cylinder. 2. Add 1 small piece of potato to a clean test tube. (Push down with stirring rod) 3. Carefully observe the rate of reaction (how rapidly the solution bubbles) as you add 2 ml of the 3% hydrogen peroxide solution into the test tube. 4. Record the reaction rate (0-5) in TABLE 2. (Remember that liver has a reaction rate of 4) 5. Clean out test tube. 6. Repeat steps 1-5 for the chicken or apple. Part 4 Effect of Temperature on Catalase Activity CAUTION: Use a test-tube holder when handling the hot test tubes. 1. Remove the test tube from the hot water bath and allow it to air cool in test tube rack. (Set aside) 2. Go to warm water bath and bring back the test tube containing the liver and the test tube containing the hydrogen peroxide to your lab table. 3. Pour the hydrogen peroxide into the test tube containing the liver 4. Record the reaction rate (0-5) in TABLE 2. 5. Repeat steps 2-4 for the test tubes in the ice water bath. 6. Return to the test tube from the boiling water bath and pour out the water. 7. Add 2 ml of hydrogen peroxide. 8. Record the reaction rate (0-5) in TABLE 2. Name_______________________________________ Period________ Date_______________ Enzyme Lab: Catalase (Pre-Lab Questions) 1. In this lab we will be testing the rate at which catalase can break down the poisonous chemical hydrogen peroxide in our bodies. Identify the enzyme, substrate, and products in this reaction. E: S: P: 2. What do we call it when an enzyme looses its conformation? __________________________________ List 2 ways this could happen. 1) 2) 3. Read the steps for each part of the lab and use that information to fill in the Flow Chart Diagram for Part 1, 2, & 4. Take note of/draw what is added to each test tube as well as the process that test tube undergoes. Be sure to indicate which test tubes end up in each temperature water bath for Part 2. PART 1 (a) Setting up the Control a) mL of (b) In order to answer the question: “Is Catalase Reusable?” b) mL of 1 What happens to the liquid after you are done with the room temperature tests: mL of PART 2 (Temperature Tests) Distilled water liver 1 Temp: 2 3 Temp: 4 5 Temp: 6 7 Temp: ICE BATH WARM BATH #1 WARM BATH #2 BOILING Test tube #’s: ____ & ____ Test tube #’s: ____ & ____ Test tube #’s: ____ & ____ Test tube #: ____ How long? Results (remember that your data & observations from Part 1 are the “room temp” data (22ºC) for both Data Table 1 & Data Table 2) _________________: _______________________________________________________________________ (“Data Table #”) (Need to state both DV and IDV in your title) _________________: _______________________________________________________________________ Post-Lab Discussion Questions 1a) When you added the enzyme Catalase to the hydrogen peroxide solution, what gas was being released? 1b) Assuming that the reaction had enough time to finish, what should the liquid be that remains in the test tube? 2a) Why are enzymes “reusable?” 2b) What was your evidence? 3a) Using your data, which type of tissue contained the most catalase? 3b) Relate your answer to 3a to the function of that type of tissue. 4) What was the optimal temperature range for the Catalase enzyme? 5a) What does boiling do to an enzyme? 5b) What does this vocabulary word mean? 5c) What is the part of the enzyme that has been affected by heat so that a reaction is not as efficient or can no longer occur? 5d) List 1 other thing that can denature enzymes that we did not test in this experiment. 6) List 3 controls used in this experiment.