5 Electrons in Atoms
advertisement
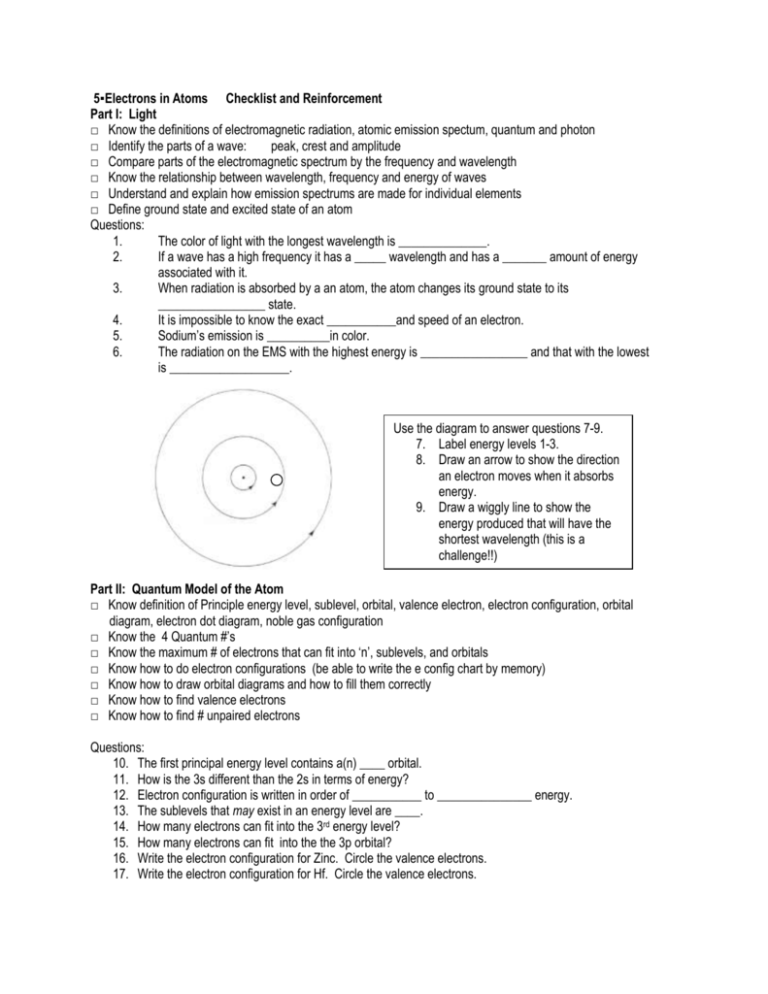
5▪Electrons in Atoms Checklist and Reinforcement Part I: Light □ Know the definitions of electromagnetic radiation, atomic emission spectum, quantum and photon □ Identify the parts of a wave: peak, crest and amplitude □ Compare parts of the electromagnetic spectrum by the frequency and wavelength □ Know the relationship between wavelength, frequency and energy of waves □ Understand and explain how emission spectrums are made for individual elements □ Define ground state and excited state of an atom Questions: 1. The color of light with the longest wavelength is ______________. 2. If a wave has a high frequency it has a _____ wavelength and has a _______ amount of energy associated with it. 3. When radiation is absorbed by a an atom, the atom changes its ground state to its _________________ state. 4. It is impossible to know the exact ___________and speed of an electron. 5. Sodium’s emission is __________in color. 6. The radiation on the EMS with the highest energy is _________________ and that with the lowest is ___________________. Use the diagram to answer questions 7-9. 7. Label energy levels 1-3. 8. Draw an arrow to show the direction an electron moves when it absorbs energy. 9. Draw a wiggly line to show the energy produced that will have the shortest wavelength (this is a challenge!!) Part II: Quantum Model of the Atom □ Know definition of Principle energy level, sublevel, orbital, valence electron, electron configuration, orbital diagram, electron dot diagram, noble gas configuration □ Know the 4 Quantum #’s □ Know the maximum # of electrons that can fit into ‘n’, sublevels, and orbitals □ Know how to do electron configurations (be able to write the e config chart by memory) □ Know how to draw orbital diagrams and how to fill them correctly □ Know how to find valence electrons □ Know how to find # unpaired electrons Questions: 10. The first principal energy level contains a(n) ____ orbital. 11. How is the 3s different than the 2s in terms of energy? 12. Electron configuration is written in order of ___________ to _______________ energy. 13. The sublevels that may exist in an energy level are ____. 14. How many electrons can fit into the 3rd energy level? 15. How many electrons can fit into the the 3p orbital? 16. Write the electron configuration for Zinc. Circle the valence electrons. 17. Write the electron configuration for Hf. Circle the valence electrons. Use the periodic table to find the valence electrons for the following: 18 a. Ca b. P c. I 19. Draw the orbital diagram for Sillicon 20. Draw the orbital diagram for the valence electrons only for Rubidium. 21. How many unpaired electrons do the following elements have? a. Li b. S c. Ga 22. Draw the electron dot diagram for Fluorine and Boron.








