Pea Lab - New Page 1
advertisement

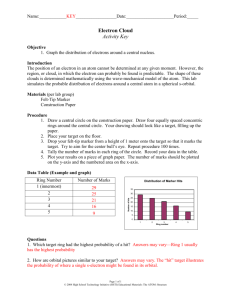
DRAFT DOCUMENT LAB 11 ELECTRON DISTRIBUTION USING PEAS Could you determine the exact position and momentum of a baseball as it soared through the air? Of course, you could—by taking timed series of snapshots of the baseball as it moved. Why then can’t scientists follow a similar procedure to determine the position and momentum of an electron? You can see a moving baseball or its image because of the light bouncing off the baseball. The effect of light on either the position or the momentum of the baseball is negligible. By contrast, an electron has such an extremely small mass that light disturbs it in an unpredictable way. How then can the position and momentum of an electron be determined? Knowledge of the behavior of electrons in the atom comes from theoretical work done in the 1920s by the German physicist Werner Heisenberg (1901-1976) and the Austrian physicist Erwin Schrodinger (1887-1961). Heisenberg postulated that it was impossible to determine exactly both the position and momentum of an electron at the same instant. Heisenberg deduced that the more precisely you know the position of an electron, the less certain you are about its momentum, and vice versa. Because its exact position and momentum can never be established at any given time, the exact path of an electron around the nucleus cannot be determined. Instead the quantum-mechanical model of the atom gives the probabilities of finding an electron in a particular region around the nucleus. In this investigation, you will model the probable locations of electrons around the nucleus of an atom. You will use peas to represent electrons to help you visualize regions of high and low electron density. OBJECTIVES When you have completed this activity, you should be able to: 1. Compare population density of a region and relate to the quantum mechanical model of the atom. 2. Observe the effects of probability on electron distribution of an atom. 3. Describe Heisenberg’s Uncertainty Principle. in order to explain the arrangement of sub-atomic particles. Adapted and edited from Chemistry Connections to Our Changing World, Prentice Hall, 1996 69 DRAFT DOCUMENT LAB 11 MATERIALS goggles 15 metric rulers 15 pairs scissors 15 compasses 1-lb bag of dried whole peas 15 plastic cups (100 peas each) PROCEDURE 15 pieces of circular filter paper, 15-25 cm in diameter 15 marking pens 15 tripod ring stands 15 sheets of butcher paper/art paper 15 beakers, 150-Ml CAUTION: Wear your goggles and lab apron at all times. 1. Put on your goggles. Fold the filter paper in half and then fold it again into quarters. Using the ruler, measure up 1.5 cm from the closed point of the paper and make a mark. Make a small hole in the bottom of the folded filter paper by cutting at the 1.5 –cm mark with the scissors. Insert the cut filter paper into the ring of the tripod ring stand to create a funnel with the small hole at the bottom. 2. Use the compass to draw a circle with a radius of 3 cm in the center of a large sheet of paper. Then draw four more concentric circles 3 cm apart, around the first circle. Number the rings 1-5, starting from the center. 3. Mark the center of the innermost circle with a large dot. Let this dot represent the nucleus of the atom. Place the ring stand so that the hole in the filter paper is exactly above the large dot, or nucleus, as shown in Figure 1. Figure 1 Adapted and edited from Chemistry Connections to Our Changing World, Prentice Hall, 1996 70 DRAFT DOCUMENT LAB 11 4. Count out 100 dried peas and place them in a plastic cup. Pinch closed the hole at the base of the filter paper and add the 100 dried peas, or “electrons,” to the filter. Let go of the filter, allowing the peas to fall through the small hole onto the target beneath the ring stand. If the peas jam up in the filter, push them gently to keep them moving. 5. Count the number of peas in each ring around the nucleus, as well as any that fall outside the rings. Record the data in the table, beginning with the innermost ring number 1. 6. Gather up the peas and place them in the plastic cup. Return all equipment to the supply area. Clean up your work area and wash your hands before leaving the lab. DATA DATA TABLE: Distribution of Peas Ring 1 2 3 4 5 Outside of rings Distance from Nucleus Number of Peas in Ring 3 cm 6 cm 9 cm 12 cm 15 cm Beyond 15 cm Adapted and edited from Chemistry Connections to Our Changing World, Prentice Hall, 1996 71 DRAFT DOCUMENT LAB 11 Using the data in the table, graph the number of peas vs. the distance from the nucleus. This can be a “best fit” curved line graph. ANALYSIS AND CONCLUSIONS 1. Judging from your graph, in which region would you be most likely to find electrons? In which region would you be least likely to find electrons? 2. Were you able to determine the exact path by which each pea (electron) arrived at its position on the target? How does this finding relate to the quantum theory? 3. Which orbital do the results of this experiment best approximate? Adapted and edited from Chemistry Connections to Our Changing World, Prentice Hall, 1996 72 DRAFT DOCUMENT LAB 11 4. What would your graph look like if you had used 200 peas instead of 100? 5. Write a brief paragraph explaining how the quantum-mechanical model of electron distribution in an atom differs from the Bohr model. 6. Why do you think that many people persist in visualizing the atom according to the out-dated Bohr model as opposed to accepting the quantum-mechanical model? EXTENSION Complete a one-page summary to compare and contrast the electron distribution probability graph to the galaxy graphic (See attached). Adapted and edited from Chemistry Connections to Our Changing World, Prentice Hall, 1996 73 DRAFT DOCUMENT LAB 11 PRE-LAB: ELECTRON DISTRIBUTION USING PEAS Read the entire laboratory investigation and the relevant pages of your text book. Then answer the questions that follow. 1. Why isn’t it possible to determine the exact path of an electron in an atom? 2. What does the quantum-mechanical model of the atom tell you about the location of an electron in an atom? 3. What do the peas represent in this investigation? What setup do you use to distribute the peas around the nucleus? 4. How will you process your data to illustrate the probabilities of finding electrons in particular region around the nucleus? Adapted and edited from Chemistry Connections to Our Changing World, Prentice Hall, 1996 74