Chapter21
advertisement

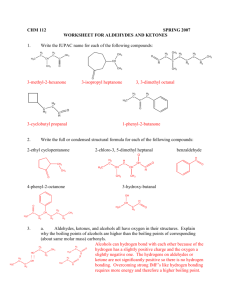
Chapter 21 Nomenclature: Highest precedence at the top Functional Group Carboxylic acid Aldehyde Ketone -COOH -CHO Alcohol thiol Amine -OH -SH -NH2 O Suffix if Higher in Precedence -oic acid -al -one Prefix if lower in Precedence oxooxo- -ol -thiol -amine hydroxy-sulfanyl -amino Naming Aldehydes: Remove the –e add an al ending. Common names: formaldehyde, acetaldehyde, benzaldehyde. O O H O H Acetaldehyde (ethanal) H O H 5-hexenal 2-phenylpropanedial Carboxyllic acids take precedence over aldehydes. You can also name aldehydes off rings. O O OH H O H O O OH H 4-Formyl-hexanoic acid 5-Oxo-pentanoic acid Cyclopentanecarbaldehyde Naming ketones: Remove the –e add an one ending. Common names: acetone, acetophenone, benzophenone. Alternate common system: Name one side, then the other and then add “ketone” O O O O 2-butanone 4-Ethyl cyclohexanone Methyl ethyl ketone (MEK) 2, 4-hexanedione Carboxyllic acids and aldehydes take precedence over ketones. O O O O O OH H 3-Oxo-hexanoic acid 3-Oxo-hexanal 1-Cyclopentyl-ethanone (?) Preparation p742 The Wittig reagent, converts ketones to alkenes. Preparation of the reagent: Ph PPh3 H3C Br Ph H + Ph BuLi P C H BrPh H + Ph + butane P C H Ph H + LiBr The Wittig reaction O H2C PPh3 + Ph3P C HH Ph Ph Ph P+ O Ph Ph Ph P Ph3PO O The Wittig can produce both cis and trans isomers although there are some conditions where mostly the cis is obtained and other conditions where trans is the predominant product. CH3CH PPh3 O + H H H Acetal and Hemiacetal formation, Nucleophillic addition, Acidic conditions. MeO OH O MeO OMe CH3OH H CH3OH H + H3C O OMe H H + + H3C O OH H3C O O H3C + O H O HOCH3 + CH3OH Sugars react to form acetals. OH O H OH H+ O Acetals can be used as protecting groups for ketones and aldehydes. H+, H2O O H+ OH OH O O Water can react with ketones in a similar way. H+, H2O HO OH O Reactions with amines. (Nucleophillic addition under buffered conditions) Primary amines form imines or Schiff bases. O CH3NH2 NCH3 mild acid CH3NH2 + H OH2 H H+ H3C N O H H + H3C N H H3C N OH H3C N H + O H Secondary amines for enamines. O (CH3)2NH H3C N CH3 mild acid H2O + H OH2 (CH3)2NH H3C H+ H3C N O CH3 H3C N OH Addition of Cyanide OH NaCN, H2O O then H2SO4 H3C C CH3 CN -:CN H C N O H3C C CH3 CN CH3 + H3C N CH3 H H3C N + O H H