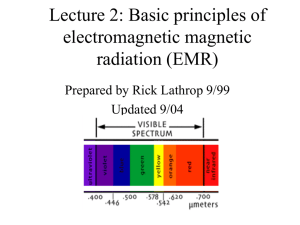
light review answers
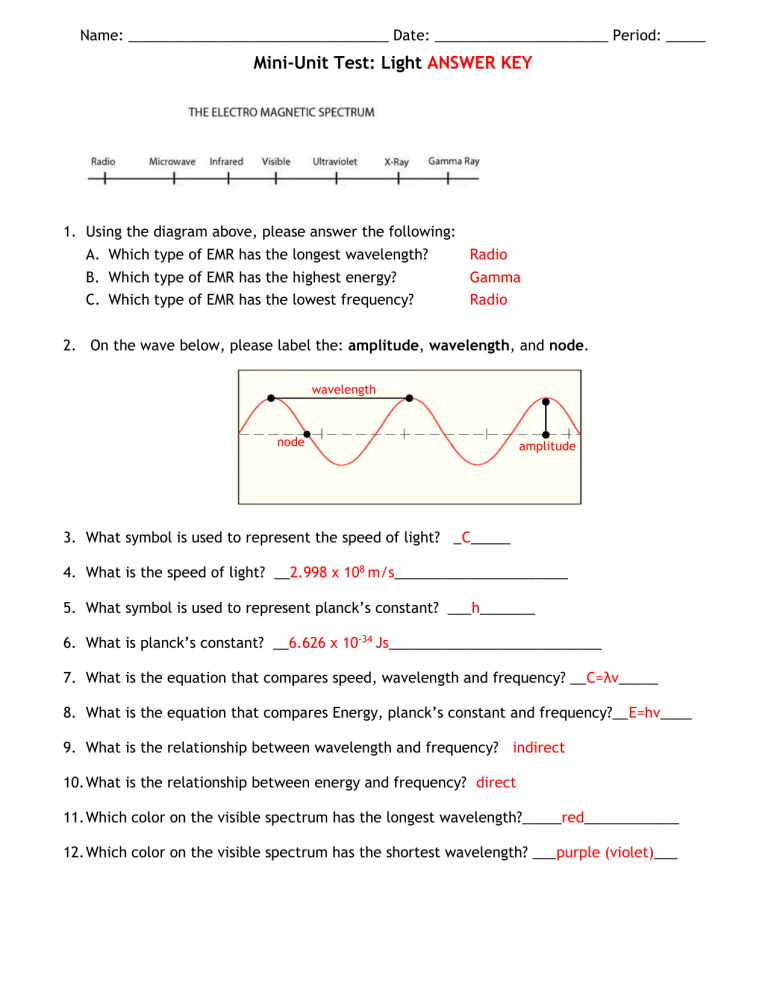
Name: _________________________________ Date: ______________________ Period: _____
Mini-Unit Test: Light ANSWER KEY
1.
Using the diagram above, please answer the following:
A.
Which type of EMR has the longest wavelength?
B.
Which type of EMR has the highest energy?
C.
Which type of EMR has the lowest frequency?
Radio
Radio
Gamma
2.
On the wave below, please label the: amplitude, wavelength, and node. wavelength node amplitude
3.
What symbol is used to represent the speed of light? _ C _____
4.
What is the speed of light? __ 2.998 x 10 8 m/s ______________________
5.
What symbol is used to represent planck’s constant? ___ h _______
6.
What is planck’s constant? __ 6.626 x 10 -34 Js ___________________________
7.
What is the equation that compares speed, wavelength and frequency? __ C=λv _____
8.
What is the equation that compares Energy, planck’s constant and frequency?__
E=hv ____
9.
What is the relationship between wavelength and frequency? indirect
10.
What is the relationship between energy and frequency? direct
11.
Which color on the visible spectrum has the longest wavelength?_____
red ____________
12.
Which color on the visible spectrum has the shortest wavelength? ___ purple (violet) ___
13.
Calculate the frequency (in Hz) of a wave whose speed is 713 m/s and a wavelength of
1.14m.
Equation: C=λv
Plug in number to equations: 713 m/s = (1.14m)(v)
Answer:____ 625 Hz __________
14.
Calculate the energy (in Joules) of a photon with a wavelength of 5.00x10
Equation: C=λv and E=hv
4 nm.
Plug in number to equations: 2.998 x 10 8 m/s= (5.00 x 10 4 nm) (v), E= (6.626 x 10 -34 Js) ( v answer
10 9 nm from first equation)
Answer:____ 3.99 x 10 -21 J _________
15.
In a hydrogen atom, when an electron jumps from an excited energy state to a more stable energy state electromagnetic radiation is ____ emitted _________ by the atom.
16.
If an AM radio station broadcasts at 995 kHz, what is the wavelength of this radiation?
Equation: C=λv
Plug in number to equations: 2.99 x 10 8 m/s = (995kHz)(1000Hz) (λ)
1 kHz
Answer:____ 3.01 x 10 2 m or 301 m _______
17.
Excited hydrogen atoms emit light in the ultraviolet at 2.47x10
single photon with this frequency?
15 Hz. What is the energy of a
Equation: E=hv
Plug in number to equations: E= (6.626 x 10 -34 Js)(2.47 x 10 15 Hz)
Answer:_____ 1.64 x 10 -18 J _____
18.
What was the experiment that Einstein did to show how light is a particle.
Photoelectric effect
19.
What did Maxwell determine about light?
Light is elelectromagnetic radiation
20.
Which scientist determined that light is considered a particle with a discrete amount of energy called quanta? Planck
21.
What is the unit for energy? Joules or J
22.
What are 2 of the 3 possible units for frequency? Hz, s -1 , or /s
23.
What instrument is used to measure the emission spectra of different gaseous elements? spectroscope