Wave Calculations Worksheet: Frequency & Energy
advertisement
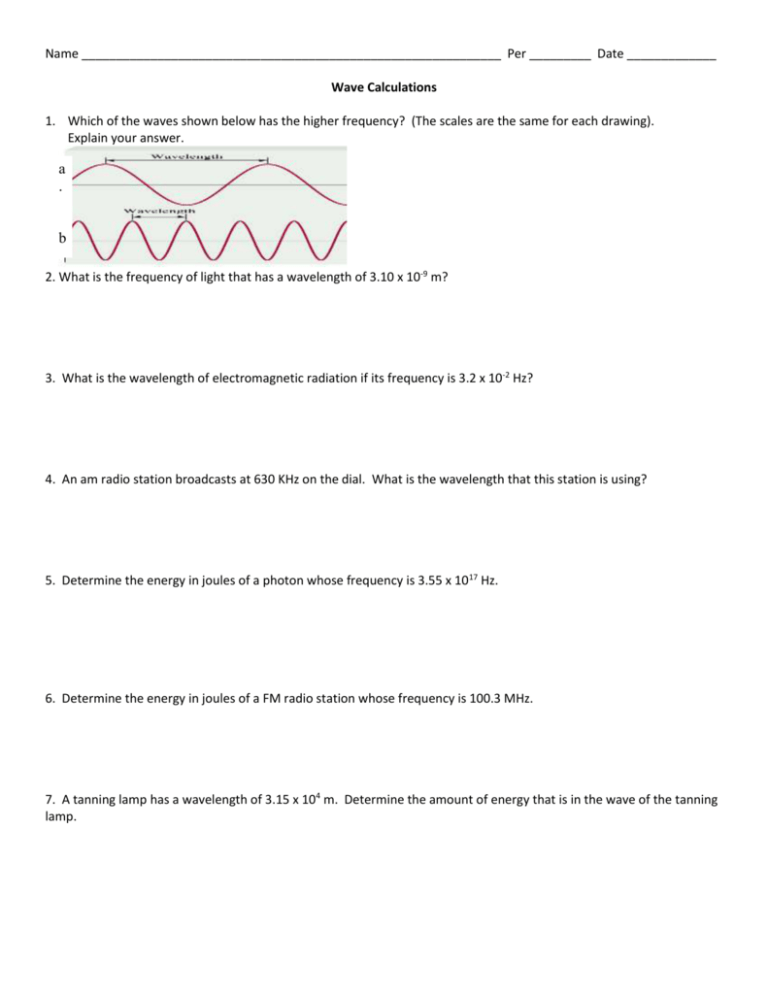
Name _____________________________________________________________ Per _________ Date _____________ Wave Calculations 1. Which of the waves shown below has the higher frequency? (The scales are the same for each drawing). Explain your answer. a . b . 2. What is the frequency of light that has a wavelength of 3.10 x 10-9 m? 3. What is the wavelength of electromagnetic radiation if its frequency is 3.2 x 10-2 Hz? 4. An am radio station broadcasts at 630 KHz on the dial. What is the wavelength that this station is using? 5. Determine the energy in joules of a photon whose frequency is 3.55 x 1017 Hz. 6. Determine the energy in joules of a FM radio station whose frequency is 100.3 MHz. 7. A tanning lamp has a wavelength of 3.15 x 104 m. Determine the amount of energy that is in the wave of the tanning lamp. 8. Consider the following energy levels of a hypothetical atom: E4 = 1.0 x 10-19 J E3 = 5.0 x 10-19 J E2 = 10 x 10-19 J E1 = 15 x 10-19 J What is the wavelength in nanometers of a photon emitted from an electronic transition from E4 to E2? 9. Consider the following energy levels of a hypothetical atom: n4 = = 1.0 x 10-19 J n3 = 5.0 x 10-19 J n2 = 10 x 10-19 J n1 = 15 x 10-19 J The wavelength in (nanometers) of a photon given off in the Lyman series is 142nm. What was the energy of the excited state?