airphoto2
advertisement

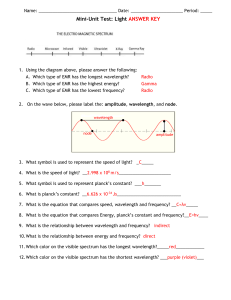
Lecture 2: Basic principles of electromagnetic magnetic radiation (EMR) Prepared by Rick Lathrop 9/99 Updated 9/04 Basic interactions between EMR and the earth surface • Reflection: specular reflection q1 = q2 • Absorption or scattering q1 q2 emission EMR re-emitted as thermal energy • Transmission Shorter ls refracted more First law of thermodynamics • Principle of conservation of energy • Energy can neither be created or destroyed, it can only be transformed • Incident E = R + A + T E R A T Adapted from Lillesand & Kiefer Remote Sensing and Image Interpretation Units of EMR measurement • Irradiance - radiant flux incident on a receiving surface from all directions, per unit surface area, W m-2 • Radiance - radiant flux emitted or scattered by a unit area of surface as measured through a solid angle, W m-2 sr-1 • Reflectance - fraction of the incident flux that is reflected by a medium Dual nature of EMR • EMR as a wave • EMR as a particle (photon) Wave nature of EMR • c=n*l where • c = 3 x 108 m/sec n = frequency, measured in hertz (cycles/sec) l = wavelength • inverse relationship between wavelength and frequency EMR wavelength vs. frequency as l gets shorter, v goes higher l = 10 mm n = 1013 Hz l = 1.0 mm n = 1014 Hz l = 0.1 mm n = 1015 Hz Wave nature of EMR: translating between wavelength and frequency • c=n*l where • c = 3 x 108 m/sec Example: n = 600 Mhz l=? n = c / l or l = c /n l = 3 x 108 m/sec / 600 x 106 hz = l = 3 x 108 m/sec / 6 x 108 hz = 0.5 m What EMR region is this wavelength? microwave The electromagnetic spectrum The electromagnetic spectrum Comparative Sizes: from subatomic to human scales Atom Nucleus Molecule Human & larger Pinhead Atom Bacteria Honeybee adapted from NY Times graphic 4/8/2003 The visible spectrum • The visible spectrum is only a tiny window • We are blind to 99.99% of the energy in the universe • One of the strengths of remote sensing is that we have created devices that allow us to see beyond the range of human vision Herschel Discovers Infrared Light • Sir Frederick Herschel (1738-1822) used a prism to split sunlight to create a spectrum and then measured the temperature of each color. He also included a control just outside the visible colors. He found to his surprise that the control actually had a higher temperature than the visible colors. Based on this observation, he concluded that there must be additional light energy beyond the visible, now known as near infrared. Incidentally if the peak of sunlight energy is in the shorter visible wavelengths, why did Herschel find the infrared to be hotter. Due to the nonlinear nature of refraction, his prism concentrated the infrared light, while dispersing the shorter wavelength visible colors. http://coolcosmos.ipac.caltech.edu/cosmic_classroom/classroom_activities/herschel_experiment.html Gee Whiz: Why do UV and not NIR rays cause sunburn? Particle nature of EMR • E = h * n = (h * c)/l where • E = energy of a photon, measured in joules • h = Planck’s constant 6.626 x 10-34 J sec • inverse relationship between wavelength and energy Why do UV and not NIR rays cause sunburn? • E = (h * c)/l = (6.626 x 10-34 J sec)(3 x 108 m/sec)/l = 19.878x10-26 J m / l • UV l = 0.3 mm – E = 19.878x10-26 J m / 0.3x10-6m = 66.26 x 10-20 J • NIR l = 0.9 mm – E = 19.878x10-26 J m / 0.9x10-6m = 22.09 x 10-20 J UV has approximately 3x the amount of energy per quanta Gee Whiz: Which emits more energy – the Sun or Earth? Relationship between temperature and EMR • M = s * T4 where • M = total radiant exittance W m-2 s = Stefan-Boltzman constant 5.6697 x 10-8 W m-2 K-4 • T = temperature in Kelvin (K) – 0oC = 273.15K Relationship between temperature and EMR • M = s * T4 where • What is M for the Sun? T= 6000K – (5.6697 x 10-8 W m-2 K-4)(6000K)4 = – (5.6697 x 10-8 W m-2 K-4)(1.296 x 1015 K4) = = 7.35 x 107 W m-2 • What is M for the Earth? T= 300K (27oC) - (5.6697 x 10-8 W m-2 K-4)(3000K)4 = 4.59 x 102 W m-2 Relationship between temperature and EMR Objects emit energy over a range of wavelengths. As the temperature of the object increases, its radiant flux increases. The wavelength of maximum flux depends on the temperature of the object. Blackbody at temperature T1 Radiant Flux T1 > T2 Blackbody at temperature T2 Wavelength Gee Whiz: Why is the outside of a candle’s flame red, while the inner flame is blue? Relationship between wavelength and temperature lm = A / T where lm = wavelength of max radiant exittance A = 2898 mm K T = temperature K Inverse relationship between temperature and lm Relationship between wavelength and temperature lm = A / T where A = 2898 mm K • What is lm for the Sun? T= 6000K lm = 2898 mm K/6000K = 0.483um lm for the sun is in the visible • What is lm for the Earth? T= 300K (27oC) lm = 2898 mm K/300K = 9.7mm lm for the earth is in the thermal IR Gee Whiz: Why do humans see in the ‘visible’ and not the NIR? Human Color Vision • Human eye contains 2 types of photoreceptors: rods and cones • Rods are more numerous and more sensitive to the amount of visible light but are not sensitive to color • 3 types of cones: roughly sensitive to blue (445nm), green (535nm) and red (575nm) For more info on color vision go to: http://hyperphysics.phy-astr.gsu.edu/hbase/vision/colviscon.html#c1 Gee Whiz: Why is the sky blue and clouds white? Atmospheric windows •Specific wavelengths where a majority of the EMR is absorbed by the atmosphere •Wavelength regions of little absorption known as atmospheric windows Graphic from http://earthobservatory.nasa.gov/Library/RemoteSensingAtmosphere/ Atmospheric interference with EMR • Shorter wavelengths strongly scattered, adding to the received signal • Longer wavelengths absorbed, subtracting from the received signal Signal decreased by absorption Signal increased by scattering Ref 0.4 0.5 0.6 0.7 0.8 1.1 um Adapted from Jensen, 1996, Introductory Digital Image Processing Why is the sky blue and clouds white? Incoming sunlight Air molecules scatter short l blue light, longer ls transmitted Rayleigh scattering Clouds scatter all ls of visible light, appear white Mie scattering Breakdown of EMR components received at the sensor Fundamental assumptions • Objects that are related can be detected, identified, and described by analyzing the energy that is reflected or emitted from them •Measurements over several bands make up a “spectral response pattern” or signature •This signature is different for different objects •This difference can be analyzed Gee Whiz: Why are plants green? Chlorophyll pigment is contained in minute structures called plastids that are found in the leave’s parenchyma cells. Chlorophyll differentially absorbs red and blue wavelengths of light, there is less absorption in the green and nearly no absorption in the near IR. Graphic from: http://iusd.k12.ca.us/uhs/cs2/leaf_cross-section.htm As light waves move from medium of one density to another (e.g., from water to air), the waves are refracted (i.e., changes direction). Graphic from: http://www.olympusmicro.com/pri mer/lightandcolor/refraction.html How plant leaves reflect light As light moves from a hydrated cell to an intercellular space it gets refracted, sometimes multiple times. Eventually, some light may be scattered back out through the upper leaf surface and some transmitted down through the leaf. NIR light (which is not absorbed) is scattered within leaf: some reflected back, some transmitted through Blue & red light strongly absorbed by chlorophyll. Green light is not as strongly absorbed Cross-section of leaf How plant leaves reflect light Sunlight B G R Incoming light Reflected light Leaf Transmitted light NIR An example-plant leaves • Chlorophyll absorbs large % of red and blue for photosynthesis- and strongly reflects in green (.55mm) • Peak reflectance in leaves in near infrared (.7-1.2mm) up to 60% of infrared energy per leaf is scattered up or down due to cell wall size, shape, leaf condition (age, stress, disease), etc. • Reflectance in Mid IR (2-4mm) influenced by water content-water absorbs IR energy, so live leaves reduce mid IR return Spectral reflectance characteristics are both spatially and temporally variable. For example, each leaf (even within the same species) is different and can change. Thus you should think of a spectral signature as more as a spectral “envelope”. Gee Whiz: Why do plants turn yellow as they senesce? As a leaf senesces, the cellular structure starts to break down and may change the NIR as well as the visible reflectance. A leaf’s chlorophyll (1) begins to break down as the leaf senesces (as in the autumn). Accessory plant pigments (such as carotenoids and anthocyanins) are also found in the leaf cells but are generally masked by chlorophyll. Without chlorophyll, these pigments dominate. Carotenoids absorb blue to blue green wavelengths and thus appear yellow to orange (2). Anthocyanins absorb blue to green wavelengths and thus appear magenta (purple) to red (3) . Graphic from: http://www.fs.fed.us/conf/fall/leafchng_nf.htm Extra Puzzler 1 • FM Radio waves have a frequency of approx. 100MHz and this energy passes through your body every second of every day with no harm done! Why? Extra Puzzler 1 • Radio wave energy passes through your body every second of every day with no harm done! Why? • E = (h * n) = (6.626 x 10-34 J sec) (100MHz) = 662.6 x 10-28 J = 6.626 x 10-26 J • Remember NIR light (which is harmless) has an quanta E of 2.209 x 10-19 J, or approx. 7 orders of magnitude higher. Extra Puzzler 2 • If a lava flow has a temperature of approximately 1000oC, what would be the best wavelength to sense it? Extra Puzzler 2 • If a lava flow has a temperature of approximately 1000oC, what would be the best wavelength to sense it? lm = A / T lm = wavelength of max radiant exittance A = 2898 mm K T = temperature K lm = A / T = 2898 mm K / 1273 K lm = 2.27 mm Which is in the short-middle infrared