Boiling Point Lab
advertisement

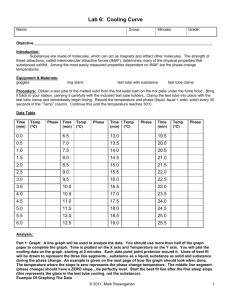
Name Class Date Freezing Point Lab Objective: To determine what happens to the properties of liquids as they reach the freezing point. Hypothesis: If a liquid cools to the freezing point then the temperature will because Materials: Procedure: Data: Time (in minutes) 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 25 30 1. 2. 3. 4. 5. Follow all SAFETY RULES. Assemble equipment according to the diagram on the board. Place the test tube containing the hot liquid into the test tube clamp. Record the starting temperature of your sample. Record your observations of your sample as it appears at the starting point, and any changes that occur as time progresses. 6. Continue to record the temperature at 1 minute intervals until the temperature is unchanged for 2 minutes, then wait 5 minutes and record again, wait 5 minutes and record again. 7. Record your observations of your sample as it appears at the finishing point. 8. Graph your data. Temperature of Sample (in oC) Observations of Sample Conclusion: (State if your hypothesis’ were correct or not and support your conclusion). Conclusion Questions: 1. In terms of the Kinetic Molecular Theory describe the process of a liquid becoming a solid. (Be sure to include terms such as attractive forces of molecules, speed of molecules and any temperature changes). 2. Explain how it is possible for a substance to be considered “frozen” and not be as cold as ice. Give an example to support your explanation. 3. What is the name of the phase change that is occurring in this lab? Where in your graph is this phase change occurring? Explain what is happening to the kinetic energy of the molecules at this point in your graph. Summary: (Write a brief 3 paragraph summary using our standard format).