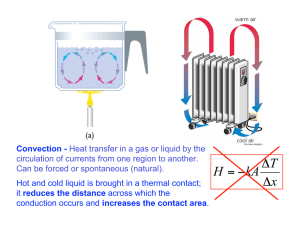
Gas pressure and the ideal gas law
advertisement

Gas pressure and the ideal gas law Assume specular collisions* Change in momentum: mv cos mv cos 2mv cos *Bold assumption – but more general calculation gives same result. Molecular Flux Element of area on the wall of the container where molecules exert a pressure due to their change in momentum upon bouncing from the wall. In time dt , molecules traveling at angle will each sweep out a volume vdtcosdA. # of molecules n volume Gas pressure and the ideal gas law Kinetic theory provides a natural interpretation of the absolute temperature of a dilute gas. Namely, the temperature is proportional to the mean kinetic energy (e ) of the gas molecules. • The mean kinetic energy is independent of pressure, volume, and the molecular species, i.e. it is the same for all molecules. 1 2 1 2 2 2 PV Nmv N mv N e NkT 3 3 2 3