Student Name ………………………… Student Number
advertisement

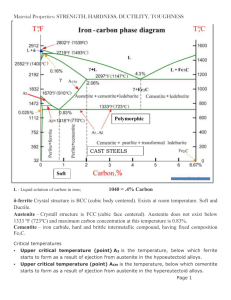
Student Name ………………………… Student Number ………………… DEPARTMENT OF MECHANICAL ENGINEERING MECH 473 Ferrous and Non-Ferrous Materials 1 hour and 15 minutes Mid-term Test No.2 Thursday July 15, 2005 1. Pearlite (total – 10 marks) Describe in metallurgical terms the nucleation, growth and morphology of pearlite in hypereutectoid and hypoeutectoid steels. To do so, answer the following. a) Why type of nucleation is involved in the formation of pearlite? (1) The nucleation of pearlite is heterogeneous. b) If the austenite is homogeneous, where will the nucleation occur? (1) If the austenite is homogeneous, the pearlite will nucleate at grain boundaries. c) If the austenite is not homogeneous where else can the pearlite nucleate? (1) If the austenite contains concentrations gradients or residual carbide particles because of a lack of time to achieve equilibrium in the –phase region, then pearlite can nucleate at these particles and defect sites within the austenite grains. d) How will the pearlite grow into the austenite at the grain boundary? (1) A nucleated pearlite colony will normally grow into only one of the two austenite grains at the boundary. It has an orientation relationship with one grain, 1, and grows into the other grain, 2. e) What is the orientation relationship of the planes and directions of the ferrite, , that is nucleating the pearlite with respect to the austenite, , if the grain boundaries are “clean”, i.e., contain no proeutectoid cementite. (1) If the grain boundaries are “clean”, then the ferrite in the pearlite will have an orientation related to the austenite grain, 1 of, 111 110a 110 111 a f) If the austenite grain boundaries contain proeutectoid cementite as found in hypereutectoid steels, what will be the orientation relationship of the cementite and ferrite in the pearlite with respect to the proeutectoid cementite? (1) The cementite in the pearlite will have the same orientation as the Fe3C in the boundary, i.e., the proeutectoid cementite, which has a complicated relationship to the 1 grain but the orientation of the ferrite is unrelated to that of the proeutectoid cementite or the 1 grain. In both of these cases, the orientation of the cementite and the ferrite in the pearlite is unrelated to the orientation of the other austenite grain, 2, into which the pearlite grows. g) Why does pearlite not grow into one austenite grain? (1) Pearlite does not grow into the austenite grain, 1, with which it has an orientation relationship because it involves a lowenergy, low-mobility interface. Pearlite does grow into the other austenite grain, 2, because the ends of the cementite and ferrite lamellae, which penetrate this grain, have high energy, high mobility interfaces. h) How will the growth of a ferrite plate affect the carbon concentration in its vicinity and what will it stimulate to nucleate at the ferrite-austenite interface? (1) The growth of a ferrite plate will increase the carbon concentration in its vicinity and thus favour the nucleation of an adjacent cementite plate at the ferrite-austenite interface. i) The growth of pearlite nodules usually have what kind of shape and why? (1) The pearlite nodules are usually spherical in shape because the rate of growth is approximately equal in all directions j) Before impinging on other colonies what is the growth rate of pearlite? (1) Before impingement the rate of pearlite growth is linear. 2. Martensite (total – 10 marks) a) What are three unique characteristics of the martensite transformation process from austenite? (1.5) The unique characteristics of the martensite transformation are: 1) it is an athermal or diffusionless transformation process, (1/2) 2) it is a displacive or shear-like transformation and (1/2) 3) it involves a change in crystal structure from fcc to bct. (1/2) b) Explain the significance of each characteristic. (1.5) The diffusionless characteristic signifies two things: 1) the amount, and 2) the composition of the old or parent phase is the same as the martensite. (1/2) The displacive or shear-like transformation signifies that a large volume of the old phase may transform at one time. (1/2) The change in crystal structure, i.e., bcc to bct, signifies that the characteristics of the martensite are different from the old phase, which makes martensite to be the primary source of hardening in steels. (1/2) c) How does the hardenability depend on carbon content? (1) The hardenability or the ability to form martensite increases with increased carbon content. d) How does the hardness depend on carbon content? (1) The hardness or strength of the martensite solely depends on the carbon content. e) How does the hardenability depend on most of the other alloying additions containing < 5% total alloy content? (1) The hardenability increases with increased alloy content for most of the alloy additions. f) How does the hardness depend on the other alloying additions? (1) The hardness or strength of the martensite does not depend on the other alloys. g) What type of martensite forms at high carbon concentrations, above what carbon content does it form, and how brittle is this martensite (does it have to be tempered in order to be used?)? (1.5) Lenticular martensite occurs in high carbon steels with > 0.4% C. They are brittle and have to be tempered in order to be used. h) How do the alloying elements other than C affect the softening of the martenistic steel during tempering? (1) The alloying elements will slow down the softening of the martenistic steel during tempering. i) In a low carbon, low alloy steel solution treated above the eutectoid temperature, how much of the austenite will transform to martensite when quenched? (1/2) 100% of the austenite in a low carbon, low alloy steel will transform to martensite when quenched. 3. Use of Phase Diagram – (total – 10 marks) a) Consider a steel with 0.2 wt%C given the phase diagram below. a) Describe all the changes in its structure as it is heated from room temperature to 850 oC. Start with its room temperature structure. (4.5) At room temperature, the 1020 steel has a ferrite plus pearlite structure (1/2). At 727 oC, the austenite phase, , forms (1/2) as the pearlite dissolves. (1/2) The iron carbide dissolves completely (1/2) and there is retained ferrite. (1/2) In the range of 727 oC to 830 oC, the ferrite and the –austenite phases co-exist. (1/2) As the temperature increases, the amount of austenite increases at the expense of the ferrite. (1/2) At 830 oC, all the ferrite transforms to austenite (1/2) and the structure at 850 oC is 100% austenite. (1/2) b) at 750 oC, write the equation to determine the equilibrium compositions and relative amounts of ferrite and austenite. Calculations of the percentages are not required. (1) At 750 oC, = 0.62%C; = 0.02%C At 750 oC and 0.2 wt%C 0.62 - 0.2 % 100 70% 0.62 - 0.02 0.2 - 0.02 % 100 30% 0.62 - 0.02 c) at 727 oC, write the equation to determine the total amount of ferrite and cementite present (1). d) write the equation to determine the amount of ferrite in the pearlite. (1) e) write the equation to determine the amount of ferrite in the specimen that is not in the pearlite (1). At the eutectic temperatu re of 727 o C has 0.02% C; Fe 3 C has 6.67% C For an 1020 steel of 0.2 wt%C 6.67 - 0.2 100 97% 6.67 - 0.02 30 - 19 (% Fe 3 C 100 3%) 6.67 - 0.02 d) The eutectoid compositio n, ie., pearlite is 0.77% C. % The fraction of (and Fe 3 C) in the pearlite is : 6.67 - 0.77 100 88.7% 6.67 - 0.02 0.77 - 0.02 (% Fe 3 C 100 11.3%) 6.67 - 0.02 e) The fraction of ferrite of the specimen and not in the pearlite is. % 0.77 - 0.2 100 76% 0.77 - 0.02 0.2 - 0.02 (% pearlite 100 24%) 0.77 - 0.02 % f) What is the relationship between the yield strength of pearlite and its interlamellar spacing? (1/2) The yield strength is an inverse linear function of the interlamellar spacing. g) How does the pearlite interlamellar spacing vary with transformation temperature from austenite. (1/2) The lower the transformation temperature from austenite, the smaller the pearlite spacing. h) Briefly describe the method used in practice to determine the pearlite interlamellar spacing. (1/2) In practice the interlamellar spacing is determined by measuring the distance between adjacent lamellae along a number of random lines marked on a polished surface. The true interlamellar spacing is taken as the mean value measured divided by 2. 4. Low Alloy Steels and TTT Diagrams – (total – 10 marks) a) What is the “ideal quench diameter” (DI)of a steel specimen? (1) The “ideal quench diameter” is the diameter of a sample that would have 50/50 P/M at its center (1/2) when cooled by an ideal quench (1/2). b) Write the equation to calculate DI for a steel consisting of 0.4%C, 0.8%Mn, 0.55%Ni, 0.1%Si, 0.55%Cr having a grain size of ASTM 7 and using the Table provided. (1) D1 = 0.213 x 3.667 x 1.201 x 1.07 x 2.188 = 2.196 inches c) If a steel with a particular quench diameter is required, rather than order one custom made, what is better to do instead? (1) If a steel is required having a particular quench diameter, it is better to examine the output from various mills and order the one, which manufactures to the required tolerance with the specification. (Consider other answers that seem reasonable although this one was given in class). d) What is microstructure produced by the cooling rates indicated on the TTT diagram given below? Indicate the any difference in morphology between c) and d) (2)? a) b) c) d) a) martensite, b) martensite and pearlite, c) fine pearlite d) course pearlite e) What does the dashed line in the TTT figure given below indicate and what microstructure is produced? (1) The dashed line refers to the critical cooling rate resulting in 100% martensite. f) What effects on the TTT curves do the alloy additions of 1% Cr and 3.5% Ni give a 0.4% C steel? (1) 1% Cr changes the TTT curves into two C-curves where the high temperature curve refers to pearlite and the low curve refers to bainite. (1/2) 3.5% Ni retards the start of the pearlite start curve. A fast water quench will give martensite, while a slow oil quench will give fine pearlite. (1/2) g) What does all this mean? Briefly describe how the tensile strength and % elongation (ductility) vary as a function of transformation temperature for the three metastable phases existing in a eutectoid composition steel. What is required of the martensite? Use a graph if it helps. (2) The graph below shows the relationship between the tensile strength and % elongation (ductility) of the steel as a function of transformation temperature. Martensite has high tensile strength but no ductility (1/2). It needs to be tempered as it is rarely used “as quenched” (1/2). Pearlite can be made to have reasonably high tensile strength (600 oC) with reasonably good ductility. (1/2) Bainite at 400 oC is in between. (1/2) h) Why is Mn an indispensible addition to hot rolled steels? (1) Mn is an indispensable addition because it reacts with the sulfur remaining in the steel to form MnS, which remains solid and deforms with the steel during hot rolling, whereas FeS is a liquid and will induce the steel to split during hot rolling. 5. Stainless Steels (1 mark each – total 10 marks) a) What is the definition of a stainless steel that differentiates it from all the other types of steel? A stainless steel is defined as a steel having 12%Cr or more as an alloying addition. b) What is the principle reason why one would want to use a stainless steel? For its corrosion resistance. c) What form of chromium carbide is normally found in stainless steels? (FeCr)4C d) Concerning the Fe-Cr phase diagram, how does Cr affect the ferritic phases (1/2) and austenite phase (1/2)? Cr is a strong ferritic phase stabilizer of the bcc structure. The delta ferrite and alpha ferrite become indistinguishable. The austenite phase is condensed into a small -loop over the temperature range of 900 – 1400 oC. e) Concerning the Fe-Ni phase diagram, how does Ni affect the austenite phase (1/2) and ferritic phases (1/2) down to room temperature? Ni – Ni is a strong gamma phase stabilizer of the FCC structure down to room temperature in Ni-Cr stainless steels such as Fe-8%Ni-18%Cr. The ferritic phases are very restricted. f) How does the gamma phase affect the brittleness of the steel at low temperatures (1/2) and why (1/2)? The gamma phase maintains the ductility of the stainless steel at low temperatures because it has the FCC structure. g) If a FCC stainless steel were to corrode, say in a marine environment, what would be the morphology of the corrosion (1/2) and why (1/2)? Steels having the FCC structure tend to form pits if and when they corrode. Dislocations form grain boundaries as a network and act as pipes for diffusion, which is required for corrosion. Dislocations in FCC materials react with each other to form extended networks that can extend deep into the material from the surface, which enables the pit morphology to be created. h) Stainless steels can suffer from the precipitation of chromium-iron carbides at grain boundaries. What do they suffer (1/2) and what are two methods that can be used to eliminate it (1/2)? Precipitation of (CrFe)4C at grain boundaries can reduce the Cr content to below 12%, which degrades the corrosion resistant property of the steel. It can be eliminated by lowering the carbon content to 0.03% or alloy the steel with Ti or Nb, which remove the carbon at TiC or NbC, without lowering the Cr content of the austenite. i) What is the predominant microstructure of the 400 series of heat treatable stainless steels (1/2)? What are the high-carbon grades used for containing 0.6-1.2%C and 16-18 % Cr, which have much harder martensite upon quenching (1/2)? The 400 series stainless steels have a martensitic structure. The high carbon grades are used for surgical instruments. j) In the thermomechanical treatment of steels, what is ausforming (1/2)? What transformation property of the steel, which is provided by its composition, is necessary for ausforming (1/2). Ausforming is the deformation of the austenite prior to the formation of martensite. It can only be used if the composition of the steel results in a bay between the pearlite and bainite reactions so that pearlite and bainite will not form during the deformation process. Afterwards the steel is quenched to room temperature to form martensite. 6. Cast Irons (total – 10 marks) What are the three constituents of cast iron? Iron, carbon and silicon. (1) What is its eutectic temperature, eutectic reaction and compositions? (1.5) eutectic 1154 oC L4.26%C 2.08%C + Cgraphite What is its eutectoid temperature, eutectoid reaction and compositions? (1.5) eutectoid 738 oC 0.68%C 0.02%C + Cgraphite Why is silicon necessary to produce cast iron? (2) Silicon is used to stabilize the formation of graphite by increasing the undercooling of the eutectoid reaction (1), which encourages the formation of ferrite and graphite phases from austenite (1). Alternatively - Si retards the formation of cementite, Fe3C, which allows more time to form graphite (1). Write the equation to convert silicon into an equivalent carbon based on the eutectic composition. (1) %Si Carbon Equivalent %C 4.3% 3 How is the addition of phosphorus treated in the carbon equivalent equation? (1) %Si %P Carbon Equivalent %C 4.3% 3 How does white cast iron obtain its name (1/2) and why is it used (1/2)? When white cast iron fractures its surface appears white. It is very hard and resistant to wear. How is the strength and ductility of gray cast iron and what is it most commonly used for? (1) Gray cast iron has low strength and low ductility and it mostly used for engine blocks. Bonus 1 mark Calculate the percentages of phases present using the Lever Rule in question 3 and the Ideal Quench Diameter in question 4.