composition and structure
advertisement

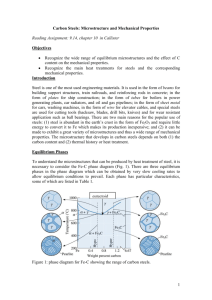
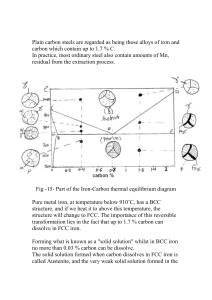
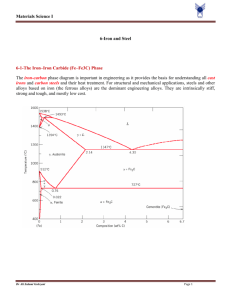
13.2 COMPOSITION AND STRUCTURE 13.2.1 Composition The chemical composition of steel is very important since it has a significant effect on the microstructure of the material and hence on its mechanical behavior and properties. Steel is basically an alloy of iron and carbon, but several elements are used in various proportions and combinations to produce different types. By definition, a steel has a maximum carbon content of 2.0%. Structural steels normally contain less than 0.30% carbon, however, and in terms of chemical composition can be classified as either plain carbon or low-alloy steels. In plain carbon steels, the amounts of carbon and manganese, the principal strengthening elements, are restricted, and other alloying elements are not normally included. In lowalloy steels, the carbon content is also restricted, and increased strength is achieved through the use of alloying elements such as nickel, chromium, and molybdenum. The alloying additions usually do not exceed a total of 8%. Carbon is by far the most important element in steel: The changes in composition (cementite or iron carbide [Fe3C] content), microstructure (pearlite content), and mechanical properties (strength and ductility, in particular) resulting from changes in the carbon content in annealed plain carbon steels are shown very clearly in Fig. 13.1. In the range of carbon contents shown in Fig. 13.1, a significant increase in strength and decrease in ductility are produced by an increase in the carbon content in the steel. Steels can be classified on the basis of composition. The most widely used system for identifying or designating carbon and alloy steels is that developed by the American Iron and Steel Institute (AISI) and the Society of Automotive Engineers (SAE). The AISI-SAE system employs a four- or five-digit designation, where the major alloying elements in a steel are indicated by the first two digits, and the amount of carbon, in hundredths of a percent, by the last two or three. Examples of several steels with their AISI-SAE designation and alloy composition are shown in Table 13.1. Structural quality steels are further classified on the basis of strength, using a system developed by the American Society for Testing and Materials (ASTM), as discussed in Section 13.6. 13.2.2 Microstructure The microstructure of metals and alloys-that is, the geometric arrangements, volume fractions, sizes, and morphologies of the constituent phases and/or grains, as observed under the microscope-plays an important part in determining the mechanical properties of the material. In general, strength depends on the nature, distribution, and size of the phases and/or grains present: Fine-grained structures are stronger than coarse-grained structures. In two-phase alloys, where one phase is hard and brittle relative to the other, optimum properties are obtained where the hard and brittle phase is uniformly distributed as isolated particles. If the brittle phase occurs as a continuous network, then fracture will follow this network, making the alloy as a whole brittle. The distribution of phases depends initially on the conditions under which the alloy solidified, but it may be varied by mechanical working and heat treatment. In a binary, metallic alloy system, the relationship between the composition of the phases present and temperature may conveniently be represented by an 2 Figure 13.1 Properties versus carbon content (annealed, plain carbon steels): (a) hardness and strength versus the amount of carbon, Fe3C, and pearlite; (b) microstructure of 0.40% C (left) and 0.80% C (right) steels (X500); and (c) ductility and toughness versus the amount of carbon, Fe3C, and pearlite (from L. H. Van Vlack, Elements of Materials Science and Engineering, 5th ed., Addison-Wesley, 1985). 3 TABLE 13.1 Examples of AISI-SAE System of Steel Designations Example AISI-SAE Type of Steel 10XX Plain carbon Designation Composition (weight %) 1020 0.18-0.23 0.30-0.60 C Mn 1040 0.37-0.44 0.60-0.90 C Mn 13XX Manganese 1340 0.38-0.43 1.60-1.90 0.15-0.30 C Mn Si 41XX Chromiummolybdenum 4140 0.30-0.43 0.75-1.00 0.15-0.30 0.80-1.10 C Mn Si Cr 0.15-0.25 Mo equilibrium or phase diagram (see Chapter 3). The following features should be noted in the iron-cementite, FeFe3C, phase diagram (Fig. 13.2): 1. Iron goes through two allotropic transformations during heating or cooling: On continued cooling from a liquid melt, it first forms a body-centered cubic (BCC) structure, then a face-centered cubic (FCC) structure, and finally another BCC structure. All three allotropes will form interstitial solid solutions with carbon, identified as delta iron (), austenite (), and ferrite (), respectively. A greater number of carbon atoms can be accommodated in the austenite than in the other two phases, since the interstitial holes in the FCC lattice are somewhat larger than those in the BCC lattices. Hence the maximum solubility of carbon in austenite is 2.0%, while it is much lower in delta iron (0.10%) and ferrite (0.025%). These solid solutions are relatively soft and ductile, but stronger than pure iron due to solid solution strengthening by the interstitial carbon atoms. 2. Cementite, or iron carbide, Fe3C, is a stoichiometric intermetallic compound formed when the solubility of carbon in solid iron is exceeded. This compound contains 6.67% C, is extremely hard and brittle, and is present in all commercial steels. The degree of dispersion strengthening and hence the properties of the steel are controlled by properly regulating the amount, size, and shape of the Fe3C phase. 3. A eutectoid reaction occurs as the austenite () cools below 727C. That is, +Fe3C. Since the two phases that form have different compositions, atoms must diffuse during the reaction: Most of the carbon in the austenite diffuses to the Fe3C, and most of the iron to the ferrite (). Since this redistribution of atoms is easiest if the diffusion distances are short, the and Fe3C grow as thin lamellae, or plates, forming a structure called pearlite (Fig. 13.3). 4 Figure 13.2 Iron-iron carbide equilibrium phase diagram (iron rich portion) (P. A. Thornton and V. J. Colangelo, Fundamentals of Engineering Materials, Prentice Hall, Inc., 1985). Figure 13.3 Photomicrograph illustrating the lamellar nature of pearlite (xl125) (Courtesy of Theresa Brassard). Since the structural steels are hypoeutectoid - that is, they contain less carbon than the eutectoid composition (0.80%) - the primary microconstituent is ferrite (a). When a hypoeutectoid alloy cools under equilibrium conditions from some temperature above, say, 900'C, the following occurs (Fig. 13.4): 5 Figure 13.4 Microstructural formation during the slow cooling of a hypoeutectoid steel from the melt (M. E Ashby and D. R. H. Jones, Engineering Materials 2, An Introduction to Microstructures, Processing and Design, Pergamon Press, 1986). Figure 13.5 Photomicrograph showing pearlite "islands" (striped regions) surrounded by primary ferrite (X8000). Courtesy of Mary Mager, Dept. of Metals and Materials Engineering, University of British Columbia. 1. Just below the A3 temperature, ferrite precipitates and grows, usually at the austenite grain boundaries; primary ferrite continues to grow until the temperature falls to A1. 6 2. At the A1 temperature, the remaining austenite is surrounded by ferrite and its composition has changed to the eutectoid composition (0.80% C); subsequent cooling causes all of this austenite to transform to pearlite by the eutectoid reaction. The final structure contains two phases-ferrite and cementite-arranged as two microconstituents: primary ferrite and pearlite (Fig. 13.5). The pearlite exists as "islands" surrounded by ferrite; this permits the alloy to be ductile, due to the continuous primary ferrite, yet strong, due to the discontinuous, dispersion-strengthened pearlite.