MSE 227 HW8 F10
advertisement

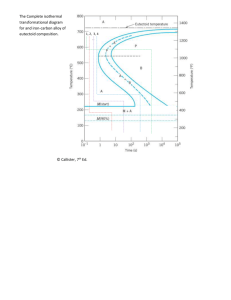
MSE 227 Due 11/2/10 Homework #8 Chapter 11: Phase Transformations 1. For the solidification of Nickel, calculate the critical radius r* and the activation free energy G* if nucleation is homogeneous. Values for the latent heat (Gv) and surface free energy, g, are -2.53 x 109 J/m3 and 0.255 J/m2, respectively. The supercooling temperature difference, T for Ni is 319 oC. 2. Suppose that a steel of eutectoid composition (0.77 wt% C) is cooled to 675 oC from 760 oC in less than 0.5 sec. and held at this temperature. (a) How long will it take for the austenite-to-pearlite reaction to go top 50% completion? To 100% completion? (b) Describe and sketch the microstructure. What will the mechanical properties be (hard, soft, strong, etc.)? 3. Using the 8isothermal transformation diagram for an iron-carbon alloy of eutectoid composition, specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that hs been submected to the following time-temperature treatments. In each case assume that the specimen begins at 760 oC and that it has been held at this temperature long enough to have achieved a complete and homogeneous austenitic structure. (a) Rapidly cool to 400 oC, hold for 500 sec., then quench to room temperature. (b) Reheat the specimen in part (a) to 700 oCfor 20 hours. (c) Cool rapdly to 665 oC, hold for 1000 sec., then quench to room temperature. (d) Rapidly cool to 350 oC, hold for 150 sec., and quench to room temperature 4. Using the TTT diagram for eutectoid steel, sketch and label the time-temperature paths to produce the following microstructures: (a) coarse pearlite (b) 50% fine pearlite and 50% bainite (c) 100% martensite (d) 100% tempered martensite Describe the mechanical properties of each of the products in (a) – (d) 5. Describe the simplest heat treatment procedure that would be used in converting a 0.76 wt% C steel from one microstructure to the other as follows: (a) Spherodite to martensite (b) Pearlite to bainite (c) Pearlite to spherodite (d) Bainite to spherodite 6. Aluminum copper alloys are precipitation hardenable. Using the Al-Cu phase diagram, (a) Specify the range of compositions over which these alloys may be precipitation hardened (b) Describe the heat treatment procedures (in terms of temperatures) that would be used to precipitation harden an alloy having a composition of your choosing, within the range given in part (a). Cu-Al Phase Diagram