9.28 Briefly explain why, upon solidification, an alloy of eutectic
advertisement

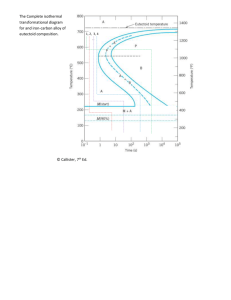
9.28 Briefly explain why, upon solidification, an alloy of eutectic composition forms a microstructure consisting of alternating layers of the two solid phases. Solution Upon solidification, an alloy of eutectic composition forms a microstructure consisting of alternating layers of the two solid phases because during the solidification atomic diffusion must occur, and with this layered configuration the diffusion path length for the atoms is a minimum. 9.46 Compute the mass fractions of α ferrite and cementite in pearlite. Solution This problem asks that we compute the mass fractions of α ferrite and cementite in pearlite. lever-rule expression for ferrite is Wα = C Fe C − C 0 3 C Fe C − Cα 3 and, since CFe C = 6.70 wt% C, C0 = 0.76 wt% C, and Cα = 0.022 wt% C 3 Wα = 6.70 − 0.76 = 0.89 6.70 − 0.022 Similarly, for cementite C 0 − Cα 0.76 − 0.022 = = 0.11 WFe C = 3 C Fe C − Cα 6.70 − 0.022 3 The 9.47 (a) What is the distinction between hypoeutectoid and hypereutectoid steels? (b) In a hypoeutectoid steel, both eutectoid and proeutectoid ferrite exist. Explain the difference between them. What will be the carbon concentration in each? Solution (a) A “hypoeutectoid” steel has a carbon concentration less than the eutectoid; on the other hand, a “hypereutectoid” steel has a carbon content greater than the eutectoid. (b) For a hypoeutectoid steel, the proeutectoid ferrite is a microconstituent that formed above the eutectoid temperature. The eutectoid ferrite is one of the constituents of pearlite that formed at a temperature below the eutectoid. The carbon concentration for both ferrites is 0.022 wt% C. 10.30 Briefly explain why fine pearlite is harder and stronger than coarse pearlite, which in turn is harder and stronger than spheroidite. Solution The hardness and strength of iron-carbon alloys that have microstructures consisting of α-ferrite and cementite phases depend on the boundary area between the two phases. The greater this area, the harder and stronger the alloy inasmuch as (1) these boundaries impede the motion of dislocations, and (2) the cementite phase restricts the deformation of the ferrite phase in regions adjacent to the phase boundaries. Fine pearlite is harder and stronger than coarse pearlite because the alternating ferrite-cementite layers are thinner for fine, and therefore, there is more phase boundary area. The phase boundary area between the sphere-like cementite particles and the ferrite matrix is less in spheroidite than for the alternating layered microstructure found in coarse pearlite. 10.31 Cite two reasons why martensite is so hard and brittle. Solution Two reasons why martensite is so hard and brittle are: (1) there are relatively few operable slip systems for the body-centered tetragonal crystal structure, and (2) virtually all of the carbon is in solid solution, which produces a solid-solution hardening effect. 11.24 Briefly explain the difference between hardness and hardenability. Solution Hardness is a measure of a material's resistance to localized surface deformation, whereas hardenability is a measure of the depth to which a ferrous alloy may be hardened by the formation of martensite. Hardenability is determined from hardness tests. 11.D15 A solution heat-treated 2014 aluminum alloy is to be precipitation hardened to have a minimum tensile strength of 450 MPa (65,250 psi) and a ductility of at least 15%EL. Specify a practical precipitation heat treatment in terms of temperature and time that would give these mechanical characteristics. Justify your answer. Solution We are asked to specify a practical heat treatment for a 2014 aluminum alloy that will produce a minimum tensile strength of 450 MPa (65,250 psi), and a minimum ductility of 15%EL. From Figure 11.27a, the following heat treating temperatures and time ranges are possible to the give the required tensile strength. Temperature (°C) Time Range (h) 260 0.02-0.2 204 0.02-10 149 3-600 121 > 35-? With regard to temperatures and times to give the desired ductility [Figure 11.27b]: Temperature (°C) Time Range (h) 260 < 0.01, > 40 204 < 0.15 149 < 10 121 < 500 From these tabulations, the following may be concluded: It is not possible to heat treat this alloy at 260°C so as to produce the desired set of properties— there is no overlap of the two sets of time ranges. At 204°C, the heat treating time would be between 0.02 and 0.15 h; times lying within this range are impractically short. At 149°C, the time would be between 3 and 10 h. Finally, at 121°C, the time range is 35 to about 500 h. 18.3 An aluminum wire 4 mm in diameter is to offer a resistance of no more than 2.5 Ω. Using the data in Table 18.1, compute the maximum wire length. Solution This problem asks that we compute, for an aluminum wire 4 mm in diameter, the maximum length such that the resistance will not exceed 2.5 Ω. From Table 18.1 for aluminum, σ = 3.8 × 107 (Ω-m)-1. If d is the diameter then, combining Equations 18.2 and 18.4 leads to l= ⎛ d ⎞2 RA = RσA = Rσπ⎜ ⎟ ⎝2⎠ ρ ⎛ 4 × 10−3 m ⎞2 = (2.5 Ω) 3.8 × 10 7 (Ω − m)−1 (π) ⎜ ⎟ = 1194 m 2 ⎝ ⎠ [ ] 10.27 Name the microstructural products of 4340 alloy steel specimens that are first completely transformed to austenite, then cooled to room temperature at the following rates: (a) 10°C/s, (b) 1°C/s, (c) 0.1°C/s, and (d) 0.01°C/s. Solution This problem asks for the microstructural products that form when specimens of a 4340 steel are continuously cooled to room temperature at several rates. Figure 10.28 is used for these determinations. (a) At a cooling rate of 10°C/s, only martensite forms. (b) At a cooling rate of 1°C/s, both martensite and bainite form. (c) At a cooling rate of 0.1°C/s, martensite, proeutectoid ferrite, and bainite form. (d) At a cooling rate of 0.01°C/s, martensite, proeutectoid ferrite, pearlite, and bainite form. 16. b 17. d