Material Science - Physics Champion
advertisement

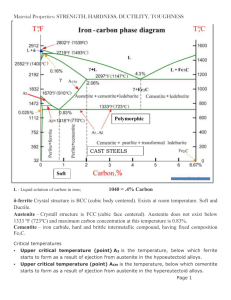
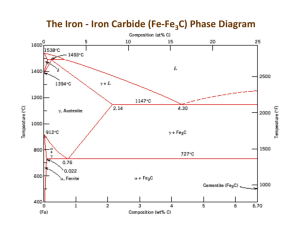
MATERIAL SCIENCE INTRODUCTION (COOLING) Observation of a pure metal cooling from the liquid state to its solid state show that it does it in a particular well defined way. As soon as the freezing point is reached, nucleii begin to form at random throughout the cooling liquid and crystal begin to form in a very special way. As soon as the nucleii are initiated the formation of crystals begins with the nucleii spreading in three directions, this process is called.... Crystal formation FORMATION HARDENING. (COOLING CURVE) Gas Evaporation/fusio n Liquid Temp Freezing Point Solid 0ºC Time HARDENING. (COOLING CURVE) Gas Liquid 910ºC Porridge/mixture Temp 720ºC Freezing Point 450ºC Crystallisation 420ºC 0ºC Time Phase Diagrams CRYSTAL STRUCTURE EQUILIBRIUM (PHASE) DIAGRAM Copper and Nickel Phase diagram Question: A liquid contain equal amounts of the alloy will begin to solidify at what temperature? 1312 ºC Question: What temperature does it become completely solid? 1248 ºC EQUILIBRIUM (PHASE) DIAGRAM Plain carbon steel equilibrium phase diagram EQUILIBRIUM (PHASE) DIAGRAM Ferrite/Iron: This is a solid solution containing no more than 0.04% carbon dissolved in a Body Centred Cubic formation lattice. Ferrite can be regarded as almost pure iron and is very soft, ductile and easily worked. = Crystal Structure 720ºC Pearlite + Ferrite Pearlite + Cementite Pearlite: This structure exist at the eutectoid of 0.83% carbon and consist of alternate layers of ferrite and Cementite. The formation of Pearlite takes place by the breakdown of Austenite below a temperature of 720ºC 0.83% Pearlite = Pearlite Crystal structure Eutectoid point Cementite: This structure exist above 0.83% carbon and is very hard and brittle and is usually found on the crystal boundary 720ºC Pearlite + Ferrite Pearlite + Cementite 0.83% Pearlite = Cementite Crystal Structure 1130ºC Austenite Pearlite + Ferrite Pearlite + Cementite Austenite: This is a solid solution of carbon, in face centre cubic and iron. The maximum carbon content is 1.7% at 1130ºC. Austenite only exist in plain carbon steels above the UCP and is a soft non-magnetic compound 1.7% 910ºC Austenite Eutectoid point Austenite + Ferrite Austenite + Cementite 720ºC Lower Critical Point (LCP) Pearlite + Ferrite Pearlite + Cementite 0.83% carbon 100% Pearlite Pearlite changes to Austenite above 720ºC TWO THINGS THAT ARE NOT ON THE PHASE DIAGRAM Martensite, most commonly refers to a very hard form of steel crystalline structure, but it can also refer to any crystal structure that is formed by displacive transformation. It includes a class of hard minerals occurring as lath- or plateshaped crystal grains. When viewed in cross section, the lenticular (lens-shaped) crystal grains are sometimes incorrectly described as Acicular, needle shape. Martensite is formed by rapid cooling (quenching) of austenite which traps carbon atoms that do not have time to diffuse out of the crystal structure. TWO THINGS THAT ARE NOT ON THE PHASE DIAGRAM Bainite: A fine non-lamellar structure, bainite commonly consists of cemenite and dislocation-rich ferrite. The high concentration of dislocations in the ferrite that are present in bainite makes this ferrite harder than it normally would be. The temperature range for transformation to bainite (250–550 °C) is between those for pearlite and martensite. When formed during continuous cooling, the cooling rate to form bainite is more rapid than that required to form pearlite, but less rapid than is required to form martensite in steels of the same composition Cooling diagram for Hardening plain carbon steel Cooling This cooling process forms crystal which in turn form grains INTRODUCTION TO GRAIN STRUCTURES Metals have a crystalline structure - this is not usually visible but can be seen on galvanized lamp posts for example. When a metal solidifies from the molten state, millions of tiny crystals start to grow through the Dendritic Growth Process. The longer the metal takes to cool the larger the crystals grow in the process. These crystals form the grains in the solid metal. Each grain is a distinct crystal with its own orientation. A crystal on a crystal. GRAIN STRUCTURES Grain structures are altered by the working of the material in its solid metallic state. In particular: - 1. Hot working and cold working 2. Heat treatment 3. Over stressing due to continued working All of these processes have an effect on grain size, grain growth and orientation of the crystal structure HOT WORKING In metalworking, rolling is a metal forming process in which metal stock is passed through a pair of rolls. Rolling and other forms of metal forming is classified according to the temperature of the metal rolled. If the temperature of the metal is above its recrystallization temperature, then the process is termed as hot rolling. If the temperature of the metal is below its recrystallization temperature, the process is termed as cold rolling. In terms of usage, hot rolling processes more tonnage than any other manufacturing process, and cold rolling processes the most tonnage out of all cold working processes. RECRYSTALLIZATION TEMPERATURE The lower limit of the hot working temperature is determined by its recrystallization temperature. As a guideline, the lower limit of the hot working temperature of a material is 0.6 times its melting temperature (on an absolute temperature scale). In our case we are going to use plain carbon steel with 0.83% carbon. What is the re-crystallisation temperature? 720ºC x 0.6 = 432ºC EFFECTS ON STRUCTURE COLD WORKING Deformed crystals EFFECTS OF COLD WORKING Breaks down the crystal structure Destroys the lattice structure Deformation occur along the crystal edges Much more pressure is required to work the material The elasticity limit is exceed Work hardening occurs when not required. Internal stress occurs known as residual stress. To combat these effects the material has to be annealed HEAT TREATMENT (ANNEALING) Annealing, in metallurgy and materials science, is a heat treatment that alters a material to increaseare its ductility and to make it more There two "softening" processes workable. commonly used when metalworking: normalizing and It involves heating material to 30 to 50ºC above its upper critical temperature, maintaining a annealing. suitable temperature, depending on the mass of the material, then of it isboth cooled very slowly, usually The objective processes is to leaving it in the furnace when switch off. soften the metal and to make it less AnnealingThis can induce ductility, soften the brittle. makes further work on material, relieve internal stresses, refine the the piecebyeasier safer. and structure making itand homogeneous, improves cold working properties. ANNEALING Deformed crystals Apply heat here SOFTENING PROCESSES (NORMALISING) Normalizing is the heating of steel to 30-50ºC above its upper critical point (UCP) followed by an air cool. The cooling is faster than annealing and this is the main difference between the two processes. This limits the grain growth to a more refined grain structure and a better quality of material. The hardness and strength of normalised steel are better than that of annealed steel but it looses out where ductility is concerned. THE IRON CARBON PHASE DIAGRAM Copy this one now Temperature in ºC Annealing and Hardening 940ºC to 960ºC 1300 1200 1100 1000 900 Normalizing 800 700 600 720ºC Upper Critical Point 910ºC 500 Lower Critical Point 400 to 450ºC 400 300 Re-crystallisation range 200 100 0.2 0.4 0.6 0.8 1.0 % of carbon in the steel 1.2 1.4 1.6 1.8 HEAT TREATMENT (HARDENING PRESENTATION) The Hall–Petch method Quenching Hardening Precipitation hardening Work hardening Solid solution strengthening Martensitic transformation hardening You must talk about the following in your presentation:1. Describe the process using photos and text. 2. The lattice formation (BCC) (FCC) etc. 3. Temperatures used in the process and why. 4 The percentage carbon in the steel 5. How the steel was cooled (speed) 6 What effect the process has on the steel (crystals) HEAT TREATMENT (HARDENING) Do not Will harden but temperatures varies according to carbon content Temperature in ºC These steels will harden but have a constant temperature 1300 1200 1100 1000 900 Hardening temperature range 940ºC to 960ºC 800 700 600 720ºC Upper Critical Point 910ºC Lower Critical Point 500 400 300 200 0.3% carbon 0.83% carbon 100 0.2 0.4 0.6 0.8 1.0 % of carbon in the steel 1.2 1.4 1.6 1.8 PLASTICS PLASTICS PLASTICS PLASTICS Thermo Plastics comprise long-chain molecules held together by weak bonds (Figure a). When heat is applied, the molecules "slide past" one another and the polymer softens. On cooling, the molecules cannot slide past each other easily and the polymer hardens PLASTICS Thermo Setting long chain molecules, however, are linked together by small molecules via strong chemical bonds, a process sometimes referred to as vulcanization (Figure b). This threedimensional network is so rigid that the molecules cannot move very much even when the polymer is heated. Thus, TSs do not soften