File - Wk 1-2
advertisement

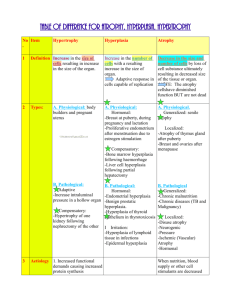
Pathology of Growth Disorders 1. Define disorders of growth and differentiation: atrophy, hyperplasia, hypertrophy, hamartoma, metaplasia, dysplasia, neoplasia. Cellular adaptations occur constantly in response to demands and requirements. They can increase or decrease cellular activity. Adaptations can be induced by: Direct stimulation of cells by the responding cells themselves or other cells. Activation of various cell surface receptors and signalling pathways New protein synthesis or cellular proliferation Switch by cells from producing one protein to another or overproducing one protein Stimuli Increase demand and external stimulation Decrease supply of nutrients and growth factors Cells changing from one type to another Cellular Response Hyperplasia or hypertrophy Atrophy Metaplasia Atrophy Shrinkage in the size of the cell Can be physiologic or pathologic Mechanisms not fully understood but hypothesised to be related to the balance between protein synthesis and degradation, especially an increase in protein degradation Physiologic atrophy is common during early development. Embryonic structures such as the notochord (elongated strip of mesodermal tissue, forms the longitudinal skeletal axis of the body. Replaced by the vertebrae later). The uterus decreases in size after parturition (process of giving birth). Pathologic atrophy depends on the underlying cause. Can be local or general. Different types are: o Decreased workload (Disuse atrophy) – complete bed rest, limb immobilisation. This rapid decrease in cell size is reversible o Loss of innervations (denervation atrophy) – damage to nerves leads to rapid atrophy of muscles o Diminished blood supply (ischaemia) – leads to progressive cell loss. In later life this leads to progressive atrophy of the brain due to atherosclerosis which narrows the blood supply. In atherosclerosis, the build up of yellow plaques of cholesterol, lipids and cellular debris causes thickening and narrowing of the lumen of the blood vessel. o Inadequate nutrition – protein-calorie malnutrition (marasmus) associated with the use of skeletal muscle as a source of energy results in marked muscle wasting (cahexia). Cahexia also seen in patients with chronic inflammatory diseases and cancer. In chronic inflammatory disease it is thought that chronic overproduction of TNF (a cytokine) is responsible for appetite suppression and muscle atrophy. o Loss of endocrine stimulation – loss of oestrogen stimulation after menopause leads to physiologic atrophy of endometrium, vaginal epithelium and breast tissue. o Aging (senile atrophy) – cell loss especially in tissues containing permanent cells such as brain and heart o Pressure – tissue compression for a prolonged period can cause atrophy, such as an enlarging benign tumour. Most probably due to ischaemic changes caused by the compromise of blood supply to those tissues. Although atrophic cells have diminished function they are not dead. However atrophy may be associated with apoptosis Mechanisms o Regulation of protein degradation plays a role. Lysosomes degrade endocytosed proteins. Ubiquitin-proteasome pathway is responsible for the degradation of cytosolic and nuclear proteins o Hormones stimulate proteasome mediated protein degradation (glucocorticoids and thyroid hormones). Insulin opposes this. o Marked increase in the number of autophagic vacuoles which are membrane bound vacuoles in a cell containing fragments of cell components destined for destruction. Lysosomes then discharge their hydrolytic contents into the vacuoles. Hyperplasia Increase in the number of cells in an organ or tissue May occur with hypertrophy (increase in the size of the cells) Hyperplasia involves an increase in mitotic activity. Hypertrophy does not. Can be physiologic or pathologic Physiologic o Hormonal hyperplasia is an increase in the functional capacity of tissues when needed. E.g. breasts develop during puberty and pregnancy and hyperplasia occurs in the pregnant uterus. o Compensatory hyperplasia is an increase in the tissue mass after damage or resection. E.g. the liver regenerates after a resection Pathologic o Most forms caused by excessive hormonal stimulation or growth factors acting on target cells o E.g. endometrial hyperplasia secondary to increased oestrogen stimulation. Hyperplasia as a response of connective tissue cells in wound healing leads to proliferating fibroblasts and blood vessels aid in repair. Growth factors are responsible for this type of hyperplasia. Hyperplasia associated with certain virus infections such as the papillomavirus that causes skin warts o Pathologic hyperplasia increases the risk of developing a neoplasia o Distinguishing feature of pathologic hyperplasia and cancer is that in cancer, the growth control mechanism becomes defective. In pathologic hyperplasia the growth regresses if the hormonal stimulation is eliminated. Mechanism o Increase local production of growth factors, growth factor receptors on cells or activation of signalling pathways o Results in cellular proliferation o Proliferation also of remaining cells and development of new cells from stem cells found in bone marrow Hypertrophy Increase in the size of the cells resulting in an increase in the size of the organ, therefore no new cells but larger cells result. No increase in mitotic activity. The increase in size is not due to cellular swelling but due to the synthesis of more structural components. Cells capable of cell division may undergo both hyperplasia and hypertrophy but nondividing cells (myocardial fibres) undergo only hypertrophy Can be physiologic or pathologic. Caused by an increase in functional demand or hormonal stimulation. Physiologic o Physiologic growth of the uterus during pregnancy due to hormone induced increase in the size of the organ results from both hyperplasia and hypertrophy o Prolactin and oestrogen causes hypertrophy of the breasts during lactation Most common stimulus for hypertrophy of muscle is increased workload. E.g. bodybuilders. The workload is shared by a greater mass of cellular components and each muscle fibre is spared excess work and so escapes injury. Mechanism o Activation of signal transduction pathways lead to induction of genes, resulting in synthesis of proteins o Induced genes include those encoding transcription factors, growth factors and vasoactive agents Hamartoma A benign mass of disorganised but mature specialised cells or tissue indigenous to the particular site. E.g. a hamartoma in the lung may consist of cartilage, blood vessels, bronchial type structures and lymphoid tissue. Metaplasia A reversible change in which one cell type is replaced by another Most commonly a change from columnar epithelial to squamous epithelial cells May represent an adaptive substitution of cells that are sensitive to stress by cells that are better able to withstand the adverse environment. E.g. a smoker’s lung which is chronically irritated, replaces the normal ciliated columnar epithelial cells of the trachea and bronchi to stratified squamous epithelial cells. Cells are better able to cope but there is a decrease in mucous production which is important in the lungs. Can also have squamous to columnar metaplasia, as occurs in Barrett oesophagus. The oesophageal squamous epithelium is replaced by intestinal like columnar cells under the influence of gastric acid. Connective tissue metaplasia is the formation of cartilage, bone or adipose tissue in tissue that does not normally contain these. E.g. bony mass in a muscle is called myositis ossificans. Mechanism o thought to arise from a reprogramming of stem cells in epithelia or from undifferentiated mesenchymal cells present in connective tissue o differentiation along a new pathway due to cytokines, growth factors, ECM components o the influences that predispose to metaplasia, if persistent may induce cancer transformation in metaplastic epithelium. Dysplasia Disordered growth Mostly seen in epithelium Loss in the uniformity of the individual cell as well as a loss in their architectural orientation Pleomorphism is common (variation in size and shape of cells) Mitosis is more abundant and often occurs in abnormal locations. E.g. in dysplastic stratified squamous epithelium, mitoses can occur in all layers, not just in the basal layer. Dysplasia does not necessarily progress to an invasive tumour and may be reversible. If dysplastic changes involve the entire thickness of the epithelium but the lesion remains confined to the normal tissue this is considered a preinvasive neoplasm and referred to as a carcinoma in situ. If the cells move beyond normal confines it is invasive. Neoplasia Literal translation is new growth Heritable genetic alterations that are passed down to progeny cells of that original neoplastic cell. The genetic changes cause excessive and unregulated proliferation that persists even after removal of the original stimulus. Usually results in an abnormal mass of tissue. 2. Describe nature, causes and complications of benign prostatic hypertrophy. Nature & Causes More correctly termed benign prostatic hyperplasia (BPH) Characterised by hyperplasia of prostatic stromal and epithelial cells resulting in formation of large nodules in the periurethral region of the prostate. NB. Stromal cells are cells of the supporting tissue or supporting matrix of an organ as distinguished from its parenchyma. E.g. Rollet stroma which contains the haemoglobin of a RBC When sufficiently enlarged they can partially or completely block the urethral canal causing obstruction or difficulty with micturition. Mechanism o Prostatic enlargement is related to the action of androgens (steroid hormone that is associated with male characteristics) o The particular androgen involved with BPH is dihydrotestosterone (DHT) o DHT is a metabolite of testosterone o testosterone DHT stimulates growth factors in both stromal and epithelial cells enlarged prostate Incidence o 20% of men 40yo o 70% by 60yo o 90% by 70yo o 50% who have microscopic evidence of BPH have clinically detectable enlargement and 50% of these develop clinical symptoms Complications Obstruction of the urethral canal, either partial or complete. Symptoms: Frequency of micturition Nocturia Difficulty in starting and stopping the stream of urine Overflow, dribbling Dysuria (painful micturition) Retention of ruine in the bladder leading to: o Distention and hypertrophy of the bladder o Infection of urine o Development of cystitis and renal infection If severely obstructed the person may require catheterisation which increases the risk of infection (invasive procedure) or developing pyelonephritis (pyogenic infection of pelvis and parenchyma of kidneys) 3. Recognise macroscopic and microscopic features of BPH Macroscopic features BPH or nodular hyperplasia originates in the inner aspect of the prostate gland in the transition zone. First nodules are almost entirely stromal cells then later epithelial nodules arise These nodules and their origins may cause compression of the urethra Nodules vary in colour and consistency Glandular nodules are yellow-pink, have soft consistency and oozes milky white prostatic fluid Fibromuscular nodules are pale-grey, tough, no exudates, less clearly demarcated from surrounding prostatic capsule Microscopic features Distinguished by nodularity due to glandular proliferation or dilatation or fibromuscular proliferation of the stroma Glandular o Aggregation of small to large cystically dilated glands lined by 2 layers, an inner columnar and an outer cuboidal or flattened epithelium o Foci of squamous metaplasia o Small area of infarction Dx of BPH cannot be made by needle biopsy alone as it only provides limited sampling (glandular Vs glandular-stromal nodules). Needle biopsies also don’t usually sample transition zone. 4. Recognise macroscopic and microscopic features of prostatic carcinoma Prostatic carcinoma is the most common type of cancer in men. There is little known about the causes however there have been suggestions of links to diet, age and race. Macroscopic features Approximately 70% of cases of prostate carcinoma arises in the peripheral zone of the gland hence it can often be palpable on DRE. Neoplastic tissue is usually gritty and firm Bony metastases often occur (lumbar spine, proximal femur, pelvis, thoracic spine, ribs) Microscopic features Well-defined glad pattern Smaller than benign glands and line by a single uniform layer of cuboidal or low columnar epithelium Appear crowded as opposed to benign glands, lacking branching and papillary infolding No basal cell layer Cytoplasm is pale to clear as in benign glands Difficult to dx on biopsy due to small amount of tissue sample available Dx made on the basis of architectural, cytological and ancillary findings and that basal cells are absent in cancer.