File - AP Physics B
advertisement

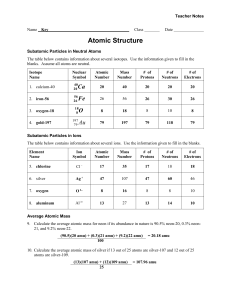
1. D—The radiation with the highest frequency (or shortest wavelength) has the highest energy per photon byE=hf. In the visible spectrum, red has the longest wavelength and violet has the shortest. Outside the visible spectrum, infrared radiation has a longer wavelength than anything visible, and ultraviolet has a shorter wavelength than anything visible. So, infrared has the smallest energy per photon, and so on up the spectrum to ultraviolet with the most energy per photon. 2. A—The total number of protons + neutrons is conserved. Before the reaction, we have one free neutron plus 235 protons and neutrons in the uranium, for a total of 236 amu. After the reaction, we have 141 amu in the barium plus 3 free neutrons for a total of 144 amu… leaving 92 AMUs for the krypton. Charge is also conserved. Before the reaction, we have a total charge of +92 from the protons in the uranium. After the reaction, we have 56 protons in the barium. Since a neutron carries no charge, the krypton must account for the remaining 36 protons. 3. E—Einstein's famous equation is written ΔE=mc2, because it is only the lost mass that is converted into energy. Since we still have a total of 236 amu after the reaction, an entire amu of mass was not converted to energy. Still, the daughter particles have kinetic energy because they move. That energy came from a very small mass difference, probably about a million times less than one amu. 4. C—In β+ emission, a positron is ejected from the nucleus. This does not change the total number of protons + neutrons, so the atomic mass A is still 15. However, charge must be conserved. The original O nucleus had a charge of +8, so the products of the decay must combine to a charge of +8. These products are a positron, charge +1, and the daughter nucleus, which must have charge +7. 5. 6. 1. Because the process is beta-plus decay, only a positron is released. As a result, the mass number A is unchanged. The atomic number is changed to Z – 1 because the atomic number of the positron is +1. 2. E=Δ mc2 6 -19 1.5 x 10 x 1.6 x 10 = Δ m (3 x 108)2 Δ m = 2.67 x 10-30 kg