Name_____________________________________________
advertisement

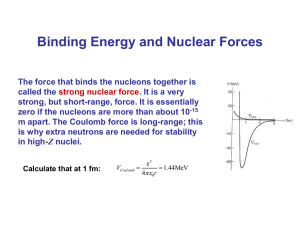
Name_____________________________________________ Date_____________________________ Modern Chemistry • CHAPTER 22 HOMEWORK N-1 (pp. 701–704) VOCABULARY Define. 1. nucleons ________________________________________________________________________________________ __________________________________________________________________________________________________ 2. nuclide _________________________________________________________________________________________ __________________________________________________________________________________________________ 3. mass defect ______________________________________________________________________________________ __________________________________________________________________________________________________ 4. nuclear binding energy _____________________________________________________________________________ __________________________________________________________________________________________________ Write true or false. ___________1. The band of stability represents the stable nuclei cluster over a range of electron-proton ratios. ___________2. The stability of a nucleus is greatest when the nucleons are in a 1:1 ratio. ___________3. 2, 20, 50, and 126 are all magic numbers. ___________4. A nuclear reaction is a change in the identity of a nucleus as a result of a change in the number of its protons. ___________5. In a transmutation, the number of protons changes, resulting in a change in the nucleus. SKILL BUILDER Complete each equation. Solve. Use masses of 1.0087 amu for the neutron, 1.00728 amu for the proton, and 5.486 x 10-4 amu for the electron. Show your work!!! 1. Calculate the binding energy per nucleon of a manganese-55 atom. The atom’s atomic mass is 54.938 amu. 2. Calculate the nuclear binding energy for a mole of oxygen-16 atoms. The atomic mass of oxygen-16 is 15.995 amu. Name_____________________________________________ Date_____________________________ Modern Chemistry • CHAPTER 22 HOMEWORK N-1 3. Calculate the nuclear binding energy of a sulfur-32 atom. The atomic mass is 31.972 amu. 4. What is the binding energy per mole for argon-40? The atomic mass is 39.962 amu. Choose the one best answer. ______1. Nuclear binding energy represents a. the energy required to break apart a nucleus. b. the measure of the stability of a nucleus. c. the energy required to bind the parts of the nucleus. d. both a and b ______2. Which nuclear symbol matches an element that has 18 protons and 21 neutrons? ______3. Which equation is correctly balanced? ______4. Which reaction is represented by this equation? a. Californium-98 reacts with boron-10 to produce lawrencium-258 and two nucleons. b. Californium-250 reacts with boron-10 to produce lawrencium-258 and two electrons. c. Californium-250 reacts with boron-5 to produce lawrencium-258 and two neutrons. d. Californium-250 reacts with boron-10 to produce lawrencium-258 and two neutrons. Name_____________________________________________ Date_____________________________ Modern Chemistry • CHAPTER 22 HOMEWORK N-1 CHAPTER 22 • HOMEWORK 22-1 VOCABULARY 1. components of atomic nuclei, protons, and neutrons 2. in nuclear chemistry, name for an atom 3. the difference between the mass of an atom and the sum of the masses of its protons, electrons, and neutrons 4. the energy released when a nucleus is formed from nucleons VOCABULARY 1. false 2. true 3. true 4. false 5. false SKILL BUILDER 1. helium-4 2. beta 3. thorium-240 4. tantalum-175 5. nobelium-254 SKILL BUILDER 1. 1.41 x 10–12 J/nucleon 2. 1.23 x 1013 J/mol 3. 4.36 x 10–11 J 4. 3.32 x 1010 kJ STANDARDIZED TEST PREP 1. d 2. b 1. a 2. b