Answers to Practice Exam #1 - Tutor
advertisement
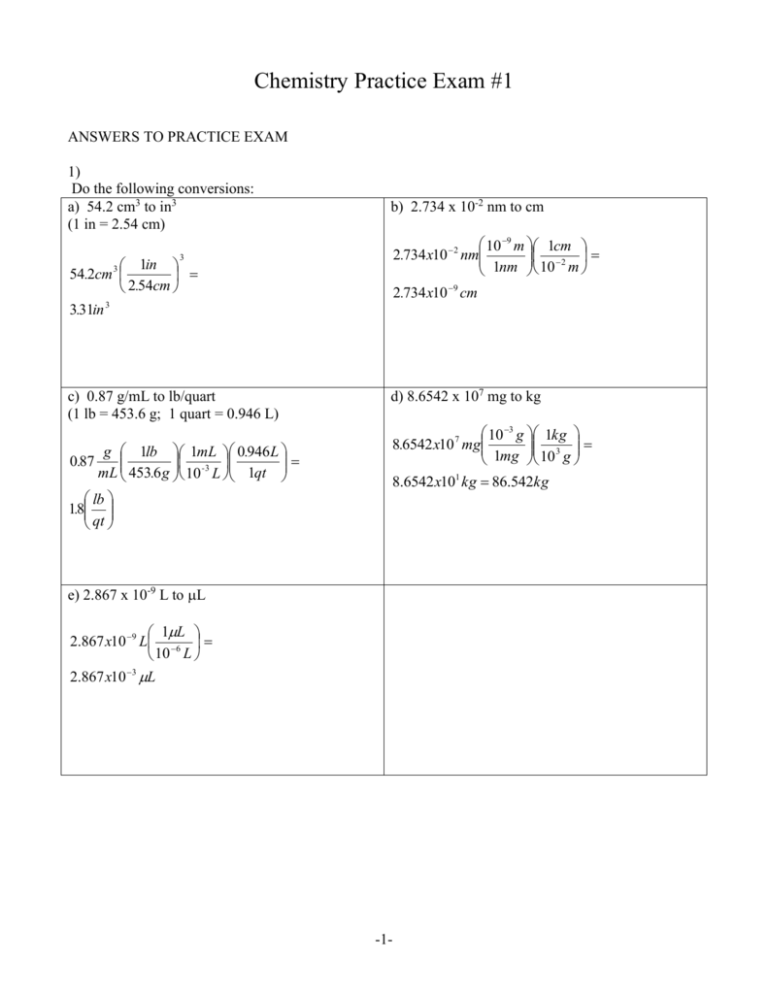
Chemistry Practice Exam #1 ANSWERS TO PRACTICE EXAM 1) Do the following conversions: a) 54.2 cm3 to in3 (1 in = 2.54 cm) b) 2.734 x 10-2 nm to cm 10 9 m 1cm 2 2.734 x10 2 nm 1nm 10 m 2.734 x10 9 cm 3 1in 54.2cm 3 2.54cm 3.31in 3 c) 0.87 g/mL to lb/quart (1 lb = 453.6 g; 1 quart = 0.946 L) 0.87 g 1lb 1mL 0.946 L mL 453.6 g 10 -3 L 1qt lb 1.8 qt d) 8.6542 x 107 mg to kg 10 3 g 1kg 3 8.6542 x10 7 mg 1mg 10 g 8.6542 x101 kg 86.542kg e) 2.867 x 10-9 L to L 1L 2.867 x10 9 L 6 10 L 3 2.867 x10 L -1- 2) Perform the following mathematical operations and express the results to the correct number of significant figures: a) 7.8132 + 0.6 + 18.24 b) (1.34 x 10-3)x(9.1 x 10-6) = 26.7 = 1.2 x 10-8 3) Answer the following True or False: a) The identity of an atom is determined by the number of electrons it has. F b) Copper (II) chloride is an ionic compound T c) Different isotopes of a given element have different masses. T d) The melting of ice is an example of a physical change. T e) Density is an example of a physical property of a substance. T 4) Co 3 has: Answer is: e) a) 27 protons, 27 electrons, 59 neutrons 59 27 b) 22 protons, 27 electrons, 37 neutrons c) 27 protons, 27 electrons, 32 neutrons d) 27 protons, 30 electrons, 32 neutrons e) 27 protons, 24 electrons, 32 neutrons -2- 5) Give the formulas of the following compounds: a) Lithium oxide b) Dinitrogen tetroxide Li2O N2O4 c) Iron (III) hydroxide d) Aluminum sulfate Fe(OH)3 Al2(SO4)3 e) Chloric acid HClO3 -3- 6) Give the names of the following compounds: a) Na2SO4 b) CuS Sodium sulfate Copper (II) sulfide c) SO2 d) (NH4)2CrO4 Sulfur dioxide Ammonium chromate e) Hydrobromic acid HBr 7) a) Gallium has two naturally occurring isotopes, 69Ga (with a mass of 68.9256 amu and a natural abundance of 60.11%) and 71Ga (with a mass of 70.9247 amu and a natural abundance of 39.89%). Calculate the average atomic mass of gallium, showing all your work. 68.9256 amu·0.6011 + 70.9247 amu·0.3989 = 69.72 amu -4- b) If 1 gram is 6.022 x 1023 atomic mass units (amu), calculate the mass in grams of one atom of 69Ga. 1g 22 68.9256amu 1.14456 x10 g 23 6 . 022 x 10 amu 8) Balance the following chemical equations: a) HI + As2S3 AsI3 + H2S b) Fe + O2 Fe2O3 6HI + As2S3 2AsI3 + 3H2S 4Fe + 3O2 2Fe2O3 c) HBr + KOH KBr + H2O d) Cs + H2O CsOH + H2 HBr + KOH KBr + H2O Balanced as is 2Cs + 2H2O 2CsOH + H2 e) C2H6O + O2 CO2 + H2O C2H6O + 3O2 2CO2 + 3H2O -5-