ME 321 Homework Solutions: Thermodynamics & Conversions
advertisement
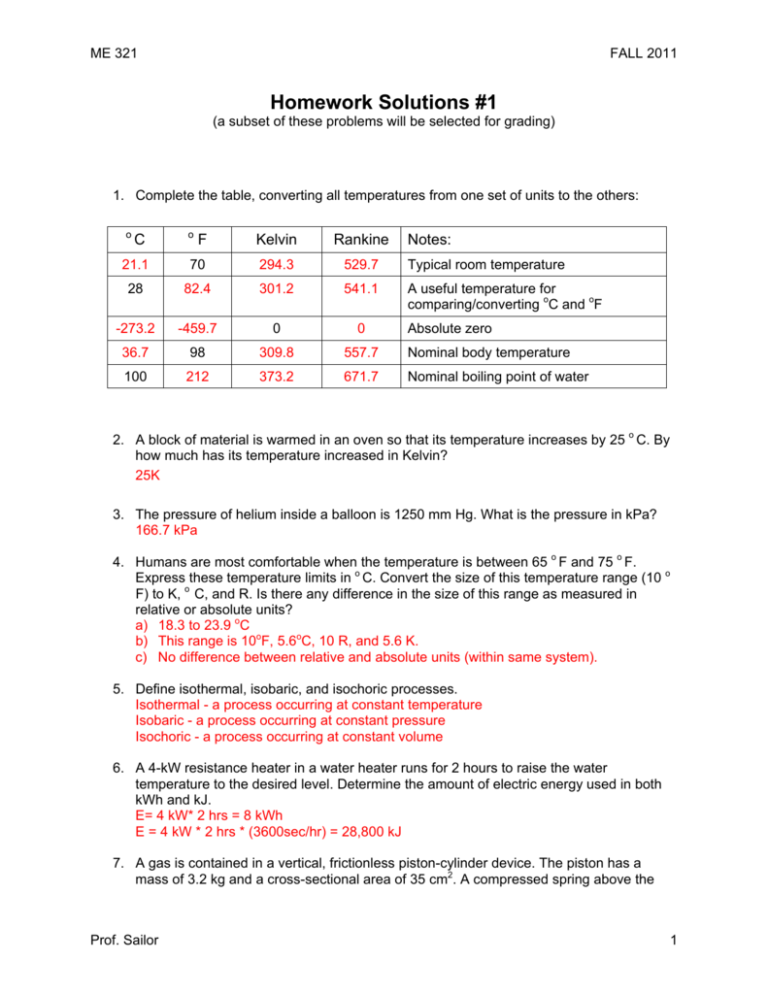
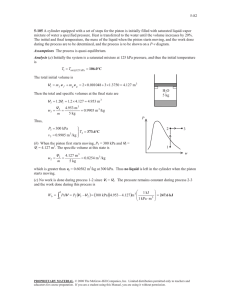
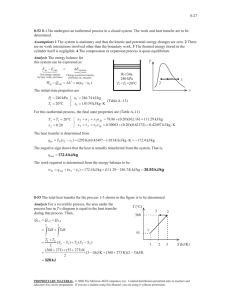
ME 321 FALL 2011 Homework Solutions #1 (a subset of these problems will be selected for grading) 1. Complete the table, converting all temperatures from one set of units to the others: o C o F Kelvin Rankine Notes: 21.1 70 294.3 529.7 Typical room temperature 28 82.4 301.2 541.1 A useful temperature for comparing/converting oC and oF -273.2 -459.7 0 0 36.7 98 309.8 557.7 Nominal body temperature 100 212 373.2 671.7 Nominal boiling point of water Absolute zero 2. A block of material is warmed in an oven so that its temperature increases by 25 o C. By how much has its temperature increased in Kelvin? 25K 3. The pressure of helium inside a balloon is 1250 mm Hg. What is the pressure in kPa? 166.7 kPa 4. Humans are most comfortable when the temperature is between 65 o F and 75 o F. Express these temperature limits in o C. Convert the size of this temperature range (10 o F) to K, o C, and R. Is there any difference in the size of this range as measured in relative or absolute units? a) 18.3 to 23.9 oC b) This range is 10oF, 5.6oC, 10 R, and 5.6 K. c) No difference between relative and absolute units (within same system). 5. Define isothermal, isobaric, and isochoric processes. Isothermal - a process occurring at constant temperature Isobaric - a process occurring at constant pressure Isochoric - a process occurring at constant volume 6. A 4-kW resistance heater in a water heater runs for 2 hours to raise the water temperature to the desired level. Determine the amount of electric energy used in both kWh and kJ. E= 4 kW* 2 hrs = 8 kWh E = 4 kW * 2 hrs * (3600sec/hr) = 28,800 kJ 7. A gas is contained in a vertical, frictionless piston-cylinder device. The piston has a mass of 3.2 kg and a cross-sectional area of 35 cm2. A compressed spring above the Prof. Sailor 1 ME 321 FALL 2011 piston exerts a force of 150 N on the piston. If the atmospheric pressure is 95 kPa, determine the pressure inside the cylinder. Forces are additive: A*Pgas = A*(Patm )+ 150N + 3.2kg*9.81m/s2 =A * 97 kN/m2 + 150 N + 31.4 N So Pgas = 95kN/m2 + (181.4 N)/A = 95 kN/m2 + 181.4N/0.0035 m2 = 95 kPa + 51,829 Pa = 146.8 kPa (note: early copy of figure gave Patm as 96 kPa, answers using this value are fine) Patm = 95 kPa Spring 150N 3.2kg gas GRADING (problems 3,4, and 7): #3: wrong = 0; correct = 2pts #4: 1 pt for part a); 1/2 pt each for each of the 3 temperatures listed in b) =5.6oC, 10 R, and 5.6 K; 1/2pt for stating that there is no difference (part c). Total of 3 possible. #7: 1 pt for sketch of system, 1 pt for eqn, 1pt for correct answer. Total of 3 possible. Total possible on this assignment = 2+3+3 = 8pts. Prof. Sailor 2