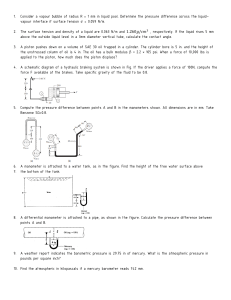
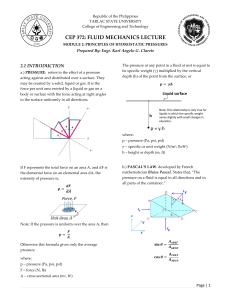
Let us assume Nitrogen as an ideal gas. From Table 1.8 (textbook
advertisement

_________________________________________________________ Let us assume Nitrogen as an ideal gas. From Table 1.8 (textbook), _________________________________________________________ RNitrogen 296.8 J/(kg K) _________________________________________________________ _________________________________________________________ Note) local standard atmosphere depends on altitude: Prescott (5,000 ft): 12.228 psi _________________________________________________________ Daytona Beach (sea-level): 14.696 psi _________________________________________________________ _________________________________________________________ _________________________________________________________ Applying the equation of state (ideal gas law): _________________________________________________________ p RT 1.5 kg/m3 296.8 J/(kg K) 25 oC 273 K = 132,670 Pa (132.67 kPa) _________________________________________________________ _________________________________________________________ Note: this is the “absolute” pressure. The purpose of this problem is to determine _________________________________________________________ the “gage” pressure. _________________________________________________________ The gage pressure is the pressure above (or below, if vacuum) the atmospheric _________________________________________________________ pressure, where the given atmospheric pressure here is: patm 97 kPa _________________________________________________________ _________________________________________________________ Therefore: _________________________________________________________ pgage pabs patm 132.67 kPa 97 kPa = 35.67 kPa _________________________________________________________ _________________________________________________________ Note that the atmospheric pressure is equal to “ZERO GAGE” pressure: patm 0 (gage) _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________ _________________________________________________________