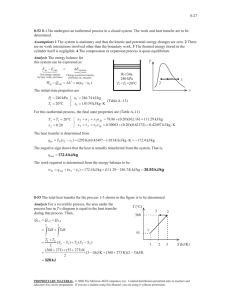
4-62 P determined.
advertisement
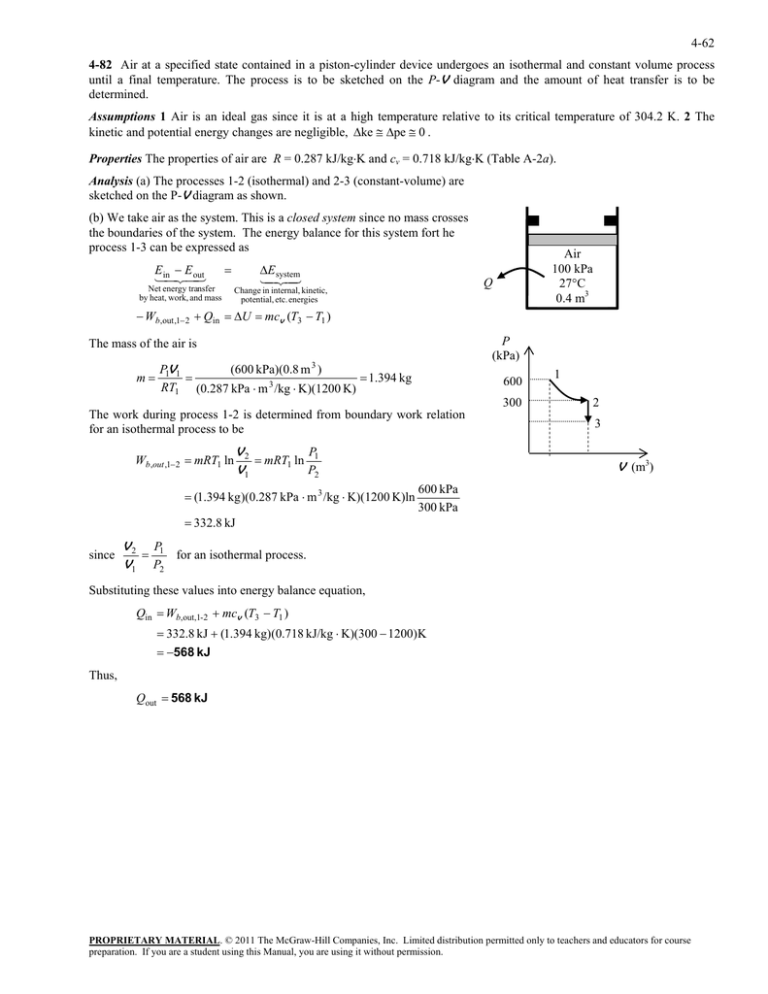
4-62 4-82 Air at a specified state contained in a piston-cylinder device undergoes an isothermal and constant volume process until a final temperature. The process is to be sketched on the P-V diagram and the amount of heat transfer is to be determined. Assumptions 1 Air is an ideal gas since it is at a high temperature relative to its critical temperature of 304.2 K. 2 The kinetic and potential energy changes are negligible, ke pe 0 . Properties The properties of air are R = 0.287 kJ/kgK and cv = 0.718 kJ/kgK (Table A-2a). Analysis (a) The processes 1-2 (isothermal) and 2-3 (constant-volume) are sketched on the P-V diagram as shown. (b) We take air as the system. This is a closed system since no mass crosses the boundaries of the system. The energy balance for this system fort he process 1-3 can be expressed as E E inout Net energy transfer by heat, work, and mass E system Air 100 kPa 27°C 0.4 m3 Q Change in internal, kinetic, potential, etc. energies Wb,out ,1 2 Qin U mcv (T3 T1 ) P (kPa) The mass of the air is m P1V1 (600 kPa)(0.8 m 3 ) 1.394 kg RT1 (0.287 kPa m 3 /kg K)(1200 K) 600 300 The work during process 1-2 is determined from boundary work relation for an isothermal process to be Wb,out ,1 2 mRT1 ln V2 P mRT1 ln 1 V1 P2 (1.394 kg )(0.287 kPa m 3 /kg K)(1200 K)ln 1 2 3 V (m3) 600 kPa 300 kPa 332.8 kJ since V 2 P1 for an isothermal process. V1 P2 Substituting these values into energy balance equation, Qin Wb,out,1-2 mcv (T3 T1 ) 332.8 kJ (1.394 kg)(0.718 kJ/kg K)(300 1200)K 568 kJ Thus, Qout 568 kJ PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.