Test 1
advertisement

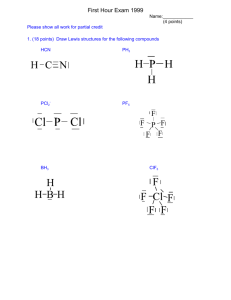
Chemistry 114 First Hour Exam Name:____________ 1. (30 points) Write the (A)Lewis structures, (B) give the electron arrangement,(C) molecular structure, (D) the bond angles, (E) the hybridization of the central atom, and (F) predict the polarity of the following compounds. (Total of 5 points/molecule) Also indicate any alternate resonance structures. Br3- POCl3 (O is central) B. Trigonal bipyramid C. Linear D. 180 E. dsp3 F. Nonpolar B. tetrahedral C. tetrahedral D. 109 E. sp3 F. Polar ClF3 SO2Cl2 (S is central) F F Cl F Other resonance with =O B. tetrahedral C. tetrahedral D. 109 E. sp3 F. Polar B. trigonal bipyramid C. T-shaped D. 90 E. dsp3 F. Polar NO- N2O4 Also resonance structures between =O and -O B. Trigonal planar C. Trigonal Planar D. 120 Resonance with NO triple bond B. Trigonal Planar C. Linear D 180 (not really defined with 2 atoms E. sp2 F. Polar? E. sp2 F. Nonpolar 1 2. (10 points) Sketch the triple bond of N2 showing the sigma(F) and pi(B) bonds that make up the triple bond. What is difference between a sigma bond and a pi bond? Sigma bonds line along a line that connects the two atoms, pi bonds line above and below (or side-to-side) of this line. Sigma bonds are slightly stronger than pi bonds 3. (10 points) Using the molecular orbital model, write electron configurations for the following diatomic species, calculate the bond order for each species, and determine which species are diamagnetic and paramagnetic F2+ 13 e F2 14 e F2p* B2p* B2p F2p F2s* F2s ¼¿¼¿ ¼¿¼¿ ¼¿ ¼¿ ¼¿ F2p* B2p* B2p F2p F2s* F2s F2p* B2p* B2p F2p F2s* F2s ¼¿¼ ¼¿¼¿ ¼¿ ¼¿ ¼¿ F215 e ¼ ¼¿¼¿ ¼¿¼¿ ¼¿ ¼¿ ¼¿ BO (8-6)/2 = 1 (8-5)/2 = 1.5 Most stable (8-7)/2 = 0.5 Least stable Diamagnetic Paramagnetic Paramagnetic 2 4. (10 points) Name the following organic chemicals: H2C CH3 H 3C H3C CH C H2 C CH3 C C CH3 H2 H 2C CH3 CH3 A 3-methylpentane B. trans-3,4-dimethyl-3-hexene O H Br Br H C C CH3 H C. Propanal D. 1,2-dibromobenzene (o- dibromobenzene) 5. (10 points) Draw structures of the following compounds A. Cyclohexene B. para-Dichlorobenzene Cl Cl C. Dimethylether D. Ethylacetate O CH3 O CH3 CH3 CH2 O C CH3 3 6. (10 points) What is the difference between an addition reaction and a substitution reaction? What types of hydrocarbons undergo these reactions? In an addition reaction a complete chemical entity is added across a double or triple bond in an alkene or an alkyne: F F + H F H C C H H H H C C H F H In a substitution reaction a Substitution is made for a H on an alkane or an aromatic system, usually with the help of a catalyst: Cl + Cl2 + HCl FeCl3 7. (10 points) Why is cyclopropane considered an unstable compound? Cyclopropane would be a ring system with 3 carbons, so the angle between carbons would o be 60 . Sp3 hybridized C normally has a bond angle of 109o. The ideal sp3 hybridized orbitals don’t line up with the atomic geometry, so there is poor overlap betweent he orbitals, and the bonds are exceptionally weak, making this an unstable compound. 8. (10 points) Give one example of a monomer and its corresponding polymer that is used in making a commercial plastic. Many different examples, the simplest is ethylene (monomer) and polyethylene (polymer) H H H H C C H C C H H H N Polyethylene (polymer) Ethylene (mononer) 4