Test 1
advertisement
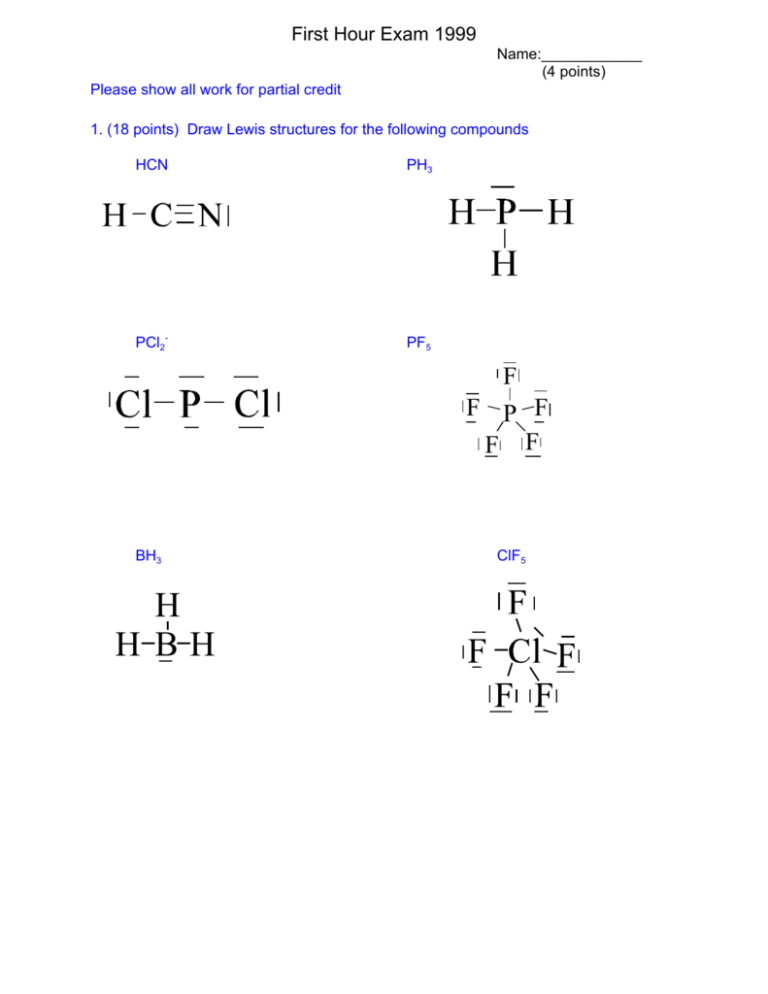
First Hour Exam 1999 Name:____________ (4 points) Please show all work for partial credit 1. (18 points) Draw Lewis structures for the following compounds HCN PH3 PCl2- PF5 F F P F F F BH3 H H BH ClF5 2 2. (18 points) Draw at least 2 non-equivalent resonance forms for each of the following molecules and evaluate the formal charge of each atom in each structure. Which is the “best” structure. ClO3- O O Cl O F.C. -Worst Cl 7-5 =+2 -O 6-7 = -1 O O Cl O Better Cl 7-6 = +1 -O 6-7 = -1 =O 6-6=0 O O Cl O Best Cl 7-7 =0 -O 6-7 = -1 =O 6-6=0 NO3You can move the double bond around, but these structures are considered equivalent. N may not exceed its octet so you can’t move into none equivalent structures FC. N = 5-4 = +1, -O 6-7 = -1, =O 6-6=0 SO3-2 O O S O F.C. Bad S 6-5 = +1 -O 6-7 = -1 O O S O Best S 6-6 = 0 -O 6-7 = -1 =O 6-6=0 O O S O Not as good S 6-7 = -1 -O 6-7 = -1 =O 6-6=0 3 3. (12 points) Write the Lewis structures and determine the molecular structure, bond angles, and polarity of the following compounds SeO3-2 KrF2 O O S O Molecular Structure : linear Bond Angles: 180o Polarity : non-polar Plus lots of resonance structures Molecular Structure : trigonal pyramid Bond Angles: <109o Polarity : Probably polar SbF5 I F 6 + F F Sb F F F Molecular Structure : trigonal bipyramid Bond Angles: 90o & 120o Polarity : non-polar Molecular Structure : octahedral Bond Angles: 90o Polarity : nonpolar 4 4. ( 12 points) Determine the Lewis structure, the hybridization of the central atom, and the bond angles in the following molecules TeF4 CF4 F F Te F F dsp2 <90 & <120 sp3 109 XeOF4 NF3 d2sp3 <90 sp3 <109 5. (12 points) Using molecular orbital theory determine the Bond Order for the following diatomic species. If the species is stable, determine whether it is paramagneitc or diamagnetic. C22_ F2p* _ _ B2p* 89 F2p 89 89 B2p 89 F2s* 89 F2s* CN+ _ F2p* _ _ B2p* _ F2p 89 89 B2p 89 F2s* 89 F2s* N2+ B.O = (8-2)/2 =3 All paired diamagnetic B.O = (6-2)/2 =2 All paired diamagnetic _ F2p* _ _ B2p* 8_ F2p B.O = (9-2)/2 =2.5 89 89 B2p 1 unpaired paramagnetic 89 F2s* 89 F2s* F2289 F2p* 89 89 B2p* 89 89 B2p B.O =0 unstable 89 F2p 89 F2s* 89 F2s* 5 6. (12 points) Draw the structure of the following compounds 2,2,4-trimethylhexane 3 none ne C-C-C=C-C-C-C-C-C 1 methylcyclopropane 2 , 3 m ethylethyl-1-octene 7. (12 points) Name the following compounds CH3 CH2 CH3 CH C CH3 CH CH3 CH CH3 CH3 CH2 CH2 CH3 3-methyl-1-pentyne C CH2 C CH2 CH2 CH3 CH3 3-ethyl-3,5,5,8-tetramethylnoname 6 2,5-dimethyl-2,4-heptadiene CH3 C CH3 CH CH C CH2 CH3 1 ethylcyclopentene CH3 CH2 CH3 7 8. (5 points Extra Credit) Karl O. Christe recently synthesized N5+. This is the first new species that contains only nitrogen to be synthesized in the last 100 years. It is only marginally stable and is highly explosive. Find a reasonable Lewis structure for N5+ and describe its molecular shape and geometry. To get you started on the right track I will tell you that it is a non-branched chain of N atoms and does not contain any ring structures.








