Naming Compounds
advertisement

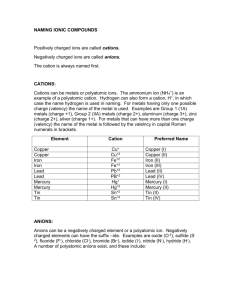
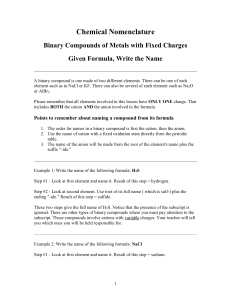
Naming Compounds 319-1 (II) name and write formulas o some common ionic compounds (both binary and complex), using the periodic table, a list of ions, and appropriate nomenclature for metal and non-metal ions Name the Following Binary Ionic Compounds • • • • • • • NaCl MgO Al4C3 Li2O Mg3N2 CaS AlCl3 • Try the worksheet on your own! Metals with more than one charge • Many metals can form more than one cation, with nearly all transition metals (the elements in Groups 3 -12 in the periodic table) being able to form more than one cation. • For example, iron can form the cations Fe2+ and Fe3+. Given the formula of an ionic compound, you can use the reverse cross-over method to determine the charge on the cation. Reverse Cross-Over Method • You can reverse the cross-over method to find the charge for the cations in a compound. Cu2S Fe2O3 PbO2* Remember that O is always -2, so lead must be +4 NiCl2 CrN * Nitrogen is -3, so Cr must be +3 HgO * Remember that O is always -2, so Hg must be +2 Multivalent Elements • There are two ways to name the cation of a transition element (an element with more than one charge). One way, is called the classical method. Classical Method • It is based on Latin names for metals that form more than one ion. • The Latin name for iron is ferrum • The Latin name for copper is cuprum • The Latin name for lead is plumbum • The –um on the end is dropped and the ending “ous” is used for the lower charge and “ic” for the higher charge. • Fill in the following table: Element iron Latin Name ferrum Ion with Ion with larger lower charge charge ferrous Fe+2 ferric Fe+3 cuprum Cu+ Cu+2 plumbum Pb+2 Pb+4 Stock System • The second, more current way to distinguish between cations was invented by German chemist, Alfred Stock. • In the stock system, you use the English name of the element. The charge of the cation is written using a Roman numeral after the name of the metal. Copy the following table. Formula FeCl3 Classical System ferric chloride FeO ferrous oxide Cu2S cuprous sulfide PbO2 plumbic oxide Stock System iron (lll) chloride • The Stock System is only used for metals that have more than one type of cation. Therefore, you do not have to use it for elements in Group 1, 2. Mainly the Stock System is used for the middle elements. • Write the chemical formula for the following compound: • copper (l) oxide • Cu+1O-2 • Cu2O • Write the chemical formula for the following compounds. • copper (l) oxide • lead (lV) bromide • iron (lll) sulfide • nickel (lll) fluoride • manganese (lV) sulfide * You will only have to know these for the test, I will not ask you for any that you have not seen. • • • • • • • • P. 113 #9 & 10 P. 115 #1&2 P. 117 #11 & 12 P. 117 #4 & 5