Materials Science Problems: Bonding, Structure, and Properties
advertisement

[
{
% ionic character = 1 " exp " (0.25)( X A " X B)
2
] }! 100
= { 1 " exp["0.25 ! (0.8 " 2.5)
2
= 51.45
2
2
6
2
6
1
K: 1s 2 s 2 p 3s 3 p 4 s
K + : 1s 2 2 s 2 2 p 6 3s 2 3 p 6
I:
1s 2 2 s 2 2 p 6 3s 2 3 p 6 4 s 2 3d 10 4 p 6 5s 2 4d 10 5 p 5
I ! : 1s 2 2 s 2 2 p 6 3s 2 3 p 6 4 s 2 3d 10 4 p 6 5s 2 4d 10 5 p 6
K + has an electron structure the same as argon.
I ! has the electron structure the same as xenon.
}! 100
2. Callister 2.15
+
!
For an Na ! Cl ion pair, attractive and repulsive energies E A and ER , respectively,
depend on the distance between the ions r, according to
ER =
EA = !
1.436
r
,
7.32 " 10!6
r8
+
!
For these expressions, energies are expressed in electron volts per Na ! Cl pair, and
r is the distance in nanometers. The net energy EN is just the sum of the two
expressions above.
(a) Superimpose on a single plot
E N ER
,
and E A versus r up to 1.0 nm.
(b) On the basis of this plot, determine (i) the equilibrium spacing r0 between the
Na + and Cl ! ions, and (ii) the magnitude of the bonding energy E0 between the two
ions.
(c) Mathematically determine the r0 and E0 values using the solutions to Problem
2.14 and compare these with the graphical results from part (b).
3. Callister 2.18a
Briefly cite the main differences between ionic, covalent, and metallic bonding.
4. Callister 2.23
Explain why hydrogen fluoride (HF) has a higher boiling temperature than hydrogen
chloride (HCl) (19.4 vs. -85°C), even though HF has a lower molecular weight.
5. Core 2.28
rA
rB
(A) Species B has the higher interatomic spacing, because
rA< rB.
(B) Species A has the higher melting temperature, because the depth of its energy well is greater.
(C) Species C has the higher coefficient of thermal expansion, because the slope of U B ( r > rB )
is less than that of U A ( r > rA )
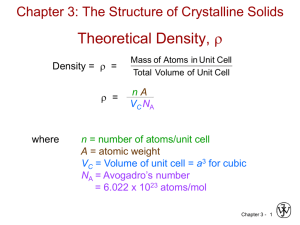
6. Core 3.02
[001]
R
a
[010]
[100]
2a 2 = 16 R 2
"=
!
m
= 2.7 g / cm3 =
v
" R3 = (
a = 2 2R
(4
atoms
1mole
)(
)(26.98 g / mole)
cell 6.022 ! 1023 atoms
(2 2 R )3
7.92 ! 10#24
1cm3
)(
) = 0.00293nm3
2.7 g / cm3 1021 nm3
! R = 0.143nm
7. Callister 3.21
Sketch a unit cell for the face-centered orthorhombic crystal structure.
[001]
(0,1,0) ! (1,0,1) = [ 1 1 1 ]or[1 1 1]
[010]
(0,1,1)-(1,0,0)= [ 1 11]or[1 1 1 ]
[100]
4 R = 3a
R
R=
2a
Farea
Farea
[110 ]
[100 ]
(4 !
=
3
a
4
3a
2
3
1
3a 2
2" !
+ 1)"R
(2" )(
)
2
2
"
R
16 = 0.833
4
4
=
=
=
2
2
2a ! a
2a
2a
2
2
1
3
(4 ! )"R
" ( a ) 2 3"
4
4
=
=
=
= 0.589 < 0.833
a!a
a2
16
4
! " ! (0.1425nm)3 ! 4atoms
M
APF =
= 3
= 0.5855
V 0.285nm ! 0.587 nm ! 0.495nm
10. Callister 3.41
Determine the Miller indices for the planes shown in the following unit cell:
+z
1
2
1
2
+y
1
2
+x