Physics 102 Practice Problems: Ch 13-15
advertisement
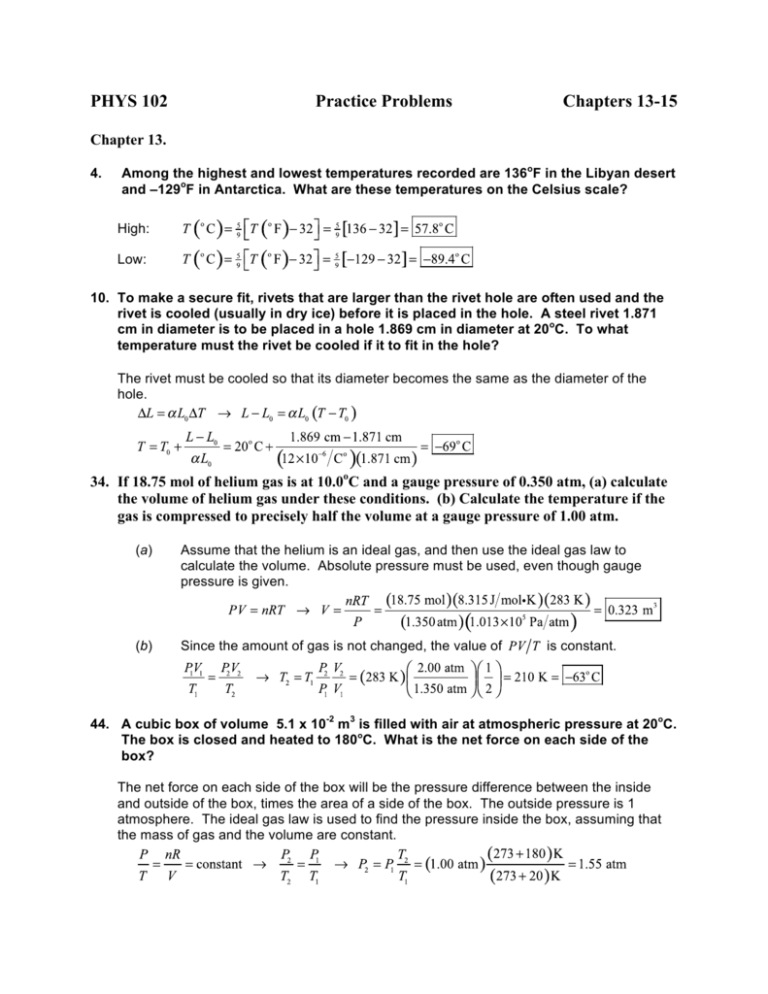
PHYS 102 Practice Problems Chapters 13-15 Chapter 13. 4. Among the highest and lowest temperatures recorded are 136oF in the Libyan desert and –129oF in Antarctica. What are these temperatures on the Celsius scale? High: Low: 10. To make a secure fit, rivets that are larger than the rivet hole are often used and the rivet is cooled (usually in dry ice) before it is placed in the hole. A steel rivet 1.871 cm in diameter is to be placed in a hole 1.869 cm in diameter at 20oC. To what temperature must the rivet be cooled if it to fit in the hole? The rivet must be cooled so that its diameter becomes the same as the diameter of the hole. 34. If 18.75 mol of helium gas is at 10.0oC and a gauge pressure of 0.350 atm, (a) calculate the volume of helium gas under these conditions. (b) Calculate the temperature if the gas is compressed to precisely half the volume at a gauge pressure of 1.00 atm. (a) Assume that the helium is an ideal gas, and then use the ideal gas law to calculate the volume. Absolute pressure must be used, even though gauge pressure is given. (b) Since the amount of gas is not changed, the value of is constant. 44. A cubic box of volume 5.1 x 10-2 m3 is filled with air at atmospheric pressure at 20oC. The box is closed and heated to 180oC. What is the net force on each side of the box? The net force on each side of the box will be the pressure difference between the inside and outside of the box, times the area of a side of the box. The outside pressure is 1 atmosphere. The ideal gas law is used to find the pressure inside the box, assuming that the mass of gas and the volume are constant. The area of a side of the box is given by The net force on a side of the box is the pressure difference times the area. Chapter 14 3. An average active person consumes about 2500 Cal a day. (Note: These are “food” calories. 1 Cal = 1 kcal = 1 cal.) (a) What is this in joules. (b) What is this in kilowatt-hours? (c) Your power company charges about a dime per kilowatt-hour. How much would your energy cost per day if you bought it from the power company? Could you feed yourself on this much money per day? (a) (b) (c) At 10 cents per day, the food energy costs . It would be practically impossible to feed yourself in the United States on this amount of money. 17. When a 290-g piece of iron at 180oC is placed in a 95-g aluminum calorimeter cup containing 250 g of glycerin at 10oC, the final temperature is observed to be 38oC. Estimate the specific heat of glycerin. The heat lost by the iron must be the heat gained by the aluminum and the glycerin. 33. One end of a 33-cm-long aluminum rod with a diameter of 2.0 cm is kept at 460oC, and he other is immersed in water at 22oC. Calculate the heat conduction rate along the rod. The heat conduction rate is given by Eq. 14-4. 41. A 100-W light bulb generates 95 W of heat, which is dissipated through a glass bulb that has a radius of 3.0 cm and is 1.0 mm thick. What is the difference in temperature between the inner and outer surfaces of the glass? This is an example of heat conduction, and the temperature difference can be calculated by Eq. 14-4. Chapter 15 4. Sketch a PV diagram of the following process: 2.0 L of ideal gas at atmospheric pressure are cooled at constant pressure to a volume of 1.0 L, and then expanded isothermally back to 2.0 L, whereupon the pressure is increase at constant volume until the original pressure is reached. 7. In an engine, an almost ideal gas is compressed adiabatically to half its volume. In doing so, 1850 J of work is done on the gas. (a) How much heat flows into or out of the gas? (b) What is the change in internal energy of the gas? (c) Does its temperature rise or fall? 17. (a) (b) Since the process is adiabatic, Use the first law of thermodynamics to find the change in internal energy. (c) Since the internal energy is proportional to the temperature, a rise in internal energy means a rise in temperature. A heat engine exhausts 8200 J of heat while performing 3200 J of useful work. What is the efficiency of this engine? The efficiency of a heat engine is given by Eq. 15-4. 23. A Carnot engine performs work at the rate of 440 kW while using 680 kcal of heat per second. If the temperature of the heat source is 570oC, at what temperature is the waste heat exhausted? This is a perfect Carnot engine, and so its efficiency is given by Eqs. 15-4 and 15-5. Equate these two expressions for the efficiency. 31. A restaurant refrigerator has a coefficient of performance of 5.0. If the temperature in the kitchen outside the refrigerator is 29oC, what is the lowest temperature that could be obtained inside the refrigerator if it were ideal? The coefficient of performance for a refrigerator is given by Eq. 15-6c, with temperatures in Kelvins. Use that expression to find the temperature inside the refrigerator. 37. What is the change in entropy of 1.00 m3 of water at 0oC when it is frozen to ice at 0oC? Heat energy is taken away from the water, so the change in entropy will be negative. The heat taken away from the water is found from . Note that 1.00 m3 of water has a mass of .











