October 15, 2012
advertisement

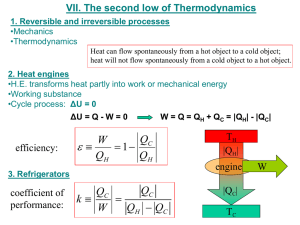
Week 4 Monday October 15, 2012 page 1 Chapter 3 (second law of thermodynamics) First law: ΔU=q+w ΔUsys + ΔUsurr = 0 closed system conservation of energy Places now restriction on transfer of energy N2 + 3H2 ⥬ 2NH3 equilibrium? Second law answers question Second law: Entropy (S) state function Clausius statement: (a cofounder of thermodynamics): It is impossible to devise an engine which working in a cycle shall produce no effect other than the transfer f heat from a colder to a hotter body. So it takes energy to operate a refrigerator Kelvin-Plank: It is impossible in a cyclic process to convert heat into work with 100% efficiency. That would be perpetual motion of the second kind. The first kind would be violating the first law. Heat engines: tao is any generic temperature scale. Hot reservoir is τH. Cold reservoir is τC. +qH is heat going into system. –qC is heat leaving system. Engine has a working substance (any substance) Takes heat, performs work Closed system Could be molten Na in a nuclear reactor Could be ammonia in refrigerator For heat engine, qH > 0, w<0, qC<0. e = efficiency of heat engine 𝑒= 𝑤𝑜𝑟𝑘 𝑜𝑢𝑡𝑝𝑢𝑡 −𝑤 |𝑤| = = 𝑒𝑛𝑒𝑟𝑔𝑦 𝑖𝑛𝑝𝑢𝑡 𝑞𝐻 𝑞𝐻 0<e<1 For cyclic process: ΔU = 0 (first law) q + w = qH + qC + w = 0 qH = -w + (-qC) 𝑠𝑜 𝑒 𝑖𝑠 𝑝𝑜𝑠𝑖𝑡𝑖𝑣𝑒 So the energy from qH is split between –w and –qC. Heat res →cyclic machine (system) → work output = q This machine violates the second law. Experimental and theoretical evidence: 1. 2. sea water → machine → work (useful) 𝑑𝑃 𝑑𝑇 = 𝛥𝐻𝑣𝑎𝑝 𝑇𝛥𝑉 𝑉 𝑖𝑠 𝑣𝑜𝑙𝑢𝑚𝑒 not possible 𝑙𝑖𝑞𝑢𝑖𝑑 ⥬ 𝑣𝑎𝑝𝑜𝑟 a. 𝑐𝑎𝑛 𝑏𝑒 𝑑𝑒𝑟𝑖𝑣𝑒𝑑 𝑓𝑟𝑜𝑚 𝑠𝑒𝑐𝑜𝑛𝑑 𝑙𝑎𝑤 b. ΔV = Vgas – Vliquid In short: 1st law says you are not going to get something for nothing (ie you cannot win) 2nd law says you cannot break even Efficiency of a heat engine: 𝑒= −𝑤 |𝑤| 𝑞𝐻 + 𝑞𝐶 𝑞 = = =1+ 𝐶 𝑞𝑤 𝑞𝐻 𝑞𝐻 𝑞𝐻 𝑞𝐶 < 0 𝑞𝐶 < 0 𝑞𝐻 > 0 𝑞𝐻 so e < 1 for a heat engine That is base on this assumption: τH and τC will not change during process, τH > τC Carnot’s principle (1825) e = efficiency of any engine working in between τH and τC e ≤ erev Carnot’s principle cannot have esuper ≥ erev