Hydrates – Hydrated and Anhydrous Compounds
advertisement

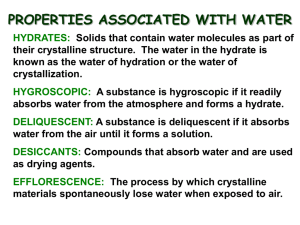
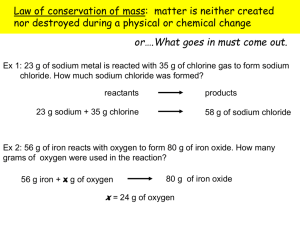
Hydrates: (Hydrated and Anhydrous compounds) Opal (SiO2) Hydrates: • Are solid crystals with trapped water molecules inside. - The name of a hydrate consists of the compound name followed by the word hydrate with a prefix indicating the number of water molecules. Example - A Hydrate is written as the ionic salt and the number of water molecules associated with one compound. - Anhydrous compounds are formed when hydrates are heated and the water of hydration is driven off. Law of Definite Proportions: • Any given compound is always made of the same elements in the same propor7on by mass. “% by Mass” & “% Composi=on”. also called, Equation __________________________ ___________________ Percent(%) Composi=on: Is, how much mass each Element, adds to a Compounds total mass (as a %). (water) (Anhydrous) Is the mass of each element (in a compound) divided by the mass of the en=re compound. __________________________________________________________________________________ Equa7on modified for Hydrates: _____Water______ X 100 Hydrated Compound = % Composi=on