Hydrate Nomenclature
advertisement

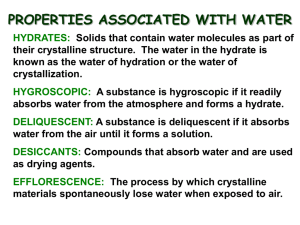
Hydrate Nomenclature Chemistry 1K Notes Addendum Naming Hydrates • Many solid compounds release water when heated at low temperatures. • Prefix Number Mono 1 Di 2 – In these compounds, called hydrates, water molecules are loosely bonded within the solid crystal at regular intervals. Tri 3 Tetra 4 A common example of a hydrate is: CuSO4 •5H2O Penta 5 – Where the compound is written first, followed by a dot (representing a weak bond) and then the number of water molecules that hydrate the compound Hexa 6 Hepta 7 Octa 8 Nona 9 Deca 10 • To name a hydrate: – Name the compound (according to the rules previously discussed) – The choose the prefix in the table to the right that indicates the number of water molecules. – Add the word “hydrate” to the end of the prefix. • A common example of a hydrate is: CuSO4 •5H2O Copper(II) sulfate pentahydrate Naming Hydrates Practice • Name each hydrate: – Li2SO4 ∙ 10H2O – Pb(OH)4 ∙ 7H2O – Cs2CO2 ∙ 2H2O lithium sulfate decahydrate lead (IV) hydroxide heptahydrate cesium carbonite dihydrate • Write the formula for each hydrate: – vanadium (III) bromide tetrahydrate – strontium nitrate pentahydrate – calcium carbonate trihydrate VBr3 ● 4H2O Sr(NO3)2 ● 5H2O CaCO3 ● 3H2O