Lab #4: Identification of an Unknown Hydrate of Copper (II) Sulfate
advertisement

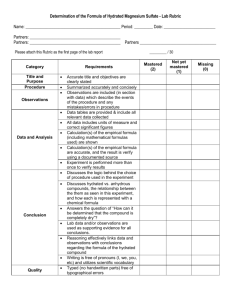
Identification of an Unknown Hydrate of Copper (II) Sulfate Background: Some compounds exist in the hydrated state. This means that in the crystal state, there are a certain number of water molecules per formula unit of the compound incorporated into the crystal structure. Purpose: To experimentally determine the formula of an unknown hydrate of copper (II) sulfate. Setup, Materials & Method: Equipment and Materials: balance clamp evaporating dish goggles laboratory burner laboratory burner lighter ring stand spatula tongs unknown hydrate (CuSO4nH2O) wire gauze Procedure: You design a detailed procedure with your lab group. Include a diagram or a detailed description of your experimental setup. Results/Data: Design a clearly-labeled data table and record all results with units. Calculations: 1. Determination of hydration number: show how you obtained your “answer” for the formula of the unknown hydrate of copper (II) sulfate. 2. Calculating molar mass: What is the molar mass of this hydrated compound? 3. Calculating percentage error: show how you calculated your % error for the hydration number. Conclusion: 1. Restate the purpose of the lab. 2. Summarize the procedure your group followed and the data you obtained. 3. State your conclusion based on your data analysis, as to the formula and name of the hydrated compound, and the molar mass of this compound. 4. Compare your results with the actual results (check with your instructor). 5. Describe possible sources of error. Questions: 1. Suppose we wish to obtain enough of this hydrate to supply 92.3 grams of anhydrous copper (II) sulfate. How many grams of the hydrated form should we weigh out? Show your work. 2. Suppose we have 150 grams of the hydrated form of this compound. How many grams of anhydrous copper (II) sulfate can we obtain from this amount? Show your work. **In addition to recording this lab in your lab notebook, YOU WILL PUBLISH THIS LAB REPORT! The following components should be included: 1. Title page 2. Abstract (summary of the entire experiment, INCLUDING your results and answer!) 3. Purpose 4. Theory (background, applicable math equations) 5. Set-up, Materials & Method, Sketch of set-up 6. Results/Data (in organized data table!!) 7. Calculations 8. Discussion/Conclusions (see above for conclusion components) 9. Answers to data analysis questions.