Polytropic Process of an Ideal Gas
advertisement

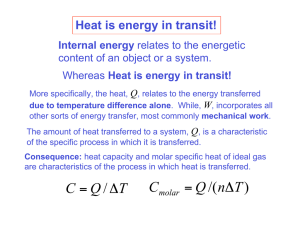
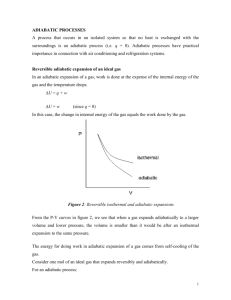
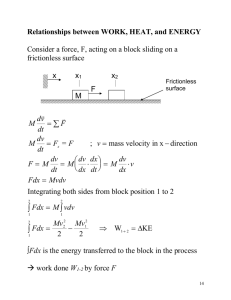
Polytropic Process of an Ideal Gas Polytropic Process of an Ideal Gas The relationship between the pressure and volume during compression or expansion of an ideal gas can be described analytically. One form of this relationship is given by the equation n • pV = constant • where n is a constant for the particular process. • A thermodynamic process described by the above equation is called a Polytropic process. For a Polytropic process between two states 1-2 • p1V = p 2 V = constant n 1 n 2 Remarks p1V = p 2 V = constant n 1 n 2 • When n=0, p = constant, and the process is a constant pressure or an isobaric process. • When n=1, pV = constant, and the process is a constant temperature or an isothermal process. • When nÆ∞, it is called an isometric process. • When n=k, it is an called isentropic process. Adiabatic Process A thermodynamic process in which there is no heat into or out of a system is called an adiabatic process. To perform an ideal adiabatic process it is necessary, that the system be surrounded by a perfect heat insulator. If a compression or expansion of a gas takes place in a short time, it would be nearly adiabatic, such as the compression stroke of a gasoline or a diesel engine. Adiabatic-Polytropic (Isentropic) Process Let an ideal gas undergo an infinitesimal adiabatic process: dQ = 0 dU = nC v dT, and dW = PdV. From the first law : dU = dQ – dW nC v dT = – PdV Taking the derivative of the ideal gas law : PV = nRT results in PdV + VdP = nRdT Eliminating dT between these two equations and using Cp – Cv = R results in : dp C p dV + =0 p CvV Adiabatic-Polytropic (Isentropic) Process Denote Cp/Cv = k, the ratio of specific heat capacities of the gas. Then Integration gives dp dV +k =0 p V ln(P) + k ln(V) = ln(constant) So pV k = constant Remarks For an adiabatic process p1V1k = p2V2k Work done during an adiabatic process: W12 = (p1V1 – p2V2)/(k-1) Alternate expression: W12 = nCv(T1-T2), for constant Cv Ideal Gas Polytropic Process From p2 ⎛ V1 ⎞ = ⎜⎜ ⎟⎟ p1 ⎝ V2 ⎠ n p1V1=nRT1 and p2V2=nRT2 n we get p2 ⎛ nRT1 / p1 ⎞ ⎛ T1 p2 ⎞ ⎛ p2 ⎞ ⎟⎟ = ⎜⎜ ⎟⎟ = ⎜⎜ ⎟⎟ = ⎜⎜ p1 ⎝ nRT2 / p2 ⎠ ⎝ T2 p1 ⎠ ⎝ p1 ⎠ T1 ⎛ p2 ⎞ or = ⎜⎜ ⎟⎟ T2 ⎝ p1 ⎠ Similarly: n T2 ⎛ V1 ⎞ = ⎜⎜ ⎟⎟ T1 ⎝ V2 ⎠ 1− n n n −1 T2 ⎛ p2 ⎞ = ⎜⎜ ⎟⎟ or T1 ⎝ p1 ⎠ n −1 n n ⎛ T1 ⎞ ⎜⎜ ⎟⎟ ⎝ T2 ⎠ n