1-δ
advertisement

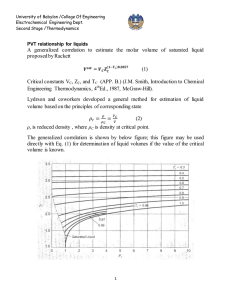
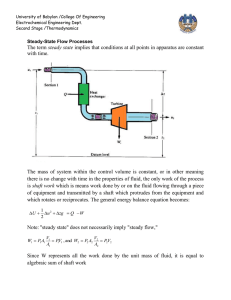
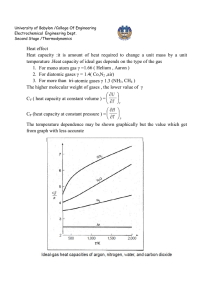
University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics IDEAL GAS The molecules making up a gas become more and more widely separated as pressure is decreased, and the volume of the molecules themselves becomes a smaller and smaller fraction of the total volume occupied by the gas. Furthermore, the forces of attraction between molecules become ever smaller because of the increasing distances between them. In the limit, as the pressure approaches zero, the molecules are separated by infinite distances. Their volumes become negligible compared with the total volume of the gas, and the intermolecular forces approach zero. At these conditions all gases are said to be ideal. PV RT R lim p 0 P // pressure (Nm-2) V- // specific volume (m3 mol-1) R// universal gas constant 8.314 Jmol-1K-1 T// temperature K PV ( PV )* 22711.6cm 3 .bar.mol 1 T T 273.15 R may be expressed in various units commonly used values are given in App. A R=8.314 J / mol. K 83.14 cm 3. bar/ mol . K University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics 0.082 lit .atm /mol . K 1.987 cal/mol. K 10.73 ft 3 .psi / mol . R CLOSED SYSTEM PROCESS 1 – Constant volume process (isometric) or isochoric T1 P 1 T2 P2 ∆U = Q = ∫ CV dt 2- Constant pressure process (isobaric) T1 V1 T2 V2 Q = ∆H = ∫ CP dt Because U = f (T) for ideal gas both H and CP also depend on temperature alone. This is evident from the definition H = U + PV or H = U + RT dH = d U + R d T CP d T = CV d T + R d T CP = CV + R This equation does not imply that CP and CV are themselves constant for an ideal gas , but only that they vary with temperature in such a way that their difference is equal to the constant R. 3- Constant temperature process (isothermal) P1 V2 P2 V1 University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics ∆U = 0 and ∆ H = 0 Q W RT ln P1 V RT ln 2 P2 V1 4- Adiabatic process ( isotropic ) : is one for which there is no heat transfer between system and surroundings dQ = 0 dU =-dW CV d T = - p dV = RT dV V dT R dV T CV V * Hint: / H U PV U RT dH dU RdT C P dT Cv dT RT CP = CV + R CP R 1 CV CV 1 R R 1 or CV CV We can write equation * as below formula: If ɤ is constant, so integration yields: ln dT dV ( 1) T V T2 V ( 1) ln 2 T1 V1 University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics T2 V1 T1 V2 Or 1 ** This equation relates temperature and volume for mechanically reversible adiabatic process involving an ideal gas with constant heat capacities . P1V1 P2V2 V PT 1 2 1 T1 T2 V2 P1T2 For ideal gas V1 V2 1 PT 2 1 P1T2 T2 P2T1 T1 P1T2 T2 P2 T1 P1 11 T2 T1 1 1 1 T1 T2 P 2 P1 1 1 T P 2 2 T1 P1 1 *** By equating equations (**) and (***) T2 V1 T1 V2 1 P 2 P1 1 University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics V 1 V2 1 P 2 P1 1 or P1V1 P2V2 PV Const. work for adiabatic process -dW = dU = CV dT W = - ∆U = - CV ∆T W CV T W RT RT1 RT2 1 1 P1V1 P2V2 1 ( because RT1 = P1 V1 and RT 2 = P2 V2) In case , V2 is not known it can be eliminated from last equation 1 1 P1V1 P2 RT1 P2 W 1 1 1 P1 1 P1 The above equations are for ideal gas with constant heat capacities for the mechanically reversible as well as adiabatic process , The process which are adiabatic but not reversible are not describes by these equations . 5- Polytropic process Since polytropic means "turning many ways " polytropic process suggests a model of some versatility. With δ a constant, it is defined as a process for which PV δ = constant TV δ-1 = constant TP (1-δ) / δ = constant Isobaric process: δ = 0. University of Babylon /College Of Engineering Electrochemical Engineering Dept. Second Stage /Thermodynamics Isothermal process: δ = 1. Adiabatic process: δ = ɤ. Isochoric process, dV/dP = V/P δ; for constant V, δ = ± ∞. It is general case and there is not specific conditions other than reversibility. For one mole , there are : dU = dQ –dW ∆ U = Q – W (first law ) dW = PdV W=∫ p dV dU = CV dT ∆U= ∫ CV dT dH= CP dT ∆H=∫ CP dT Q cannot be determined directly , but must be obtained through first law dQ = CV dT +PdV Q = ∫ CV dT + ∫ p dV Work must calculated directly from integral pdV. These equations has been derived for mechanically reversible , non flow process. The work for irreversible process is calculated by two steps procedure by : 1- W is determined for a mechanically reversible process accomplishes the same change of state . 2- The is result is multiplied or divided by an efficiency a. W irr = W rev * efficiency ( turbine - expansion ) b. W irr = W rev / efficiency ( compressor – compression )