1296221883L- Unit 20- B- Adiabatic processes2
advertisement
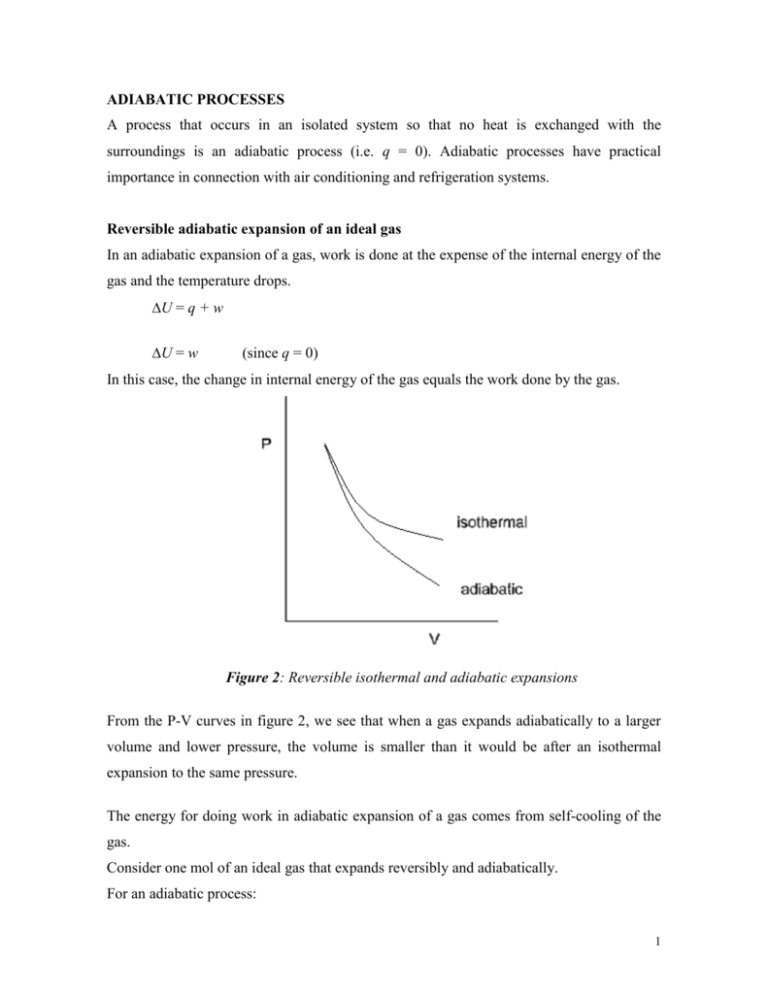
ADIABATIC PROCESSES A process that occurs in an isolated system so that no heat is exchanged with the surroundings is an adiabatic process (i.e. q = 0). Adiabatic processes have practical importance in connection with air conditioning and refrigeration systems. Reversible adiabatic expansion of an ideal gas In an adiabatic expansion of a gas, work is done at the expense of the internal energy of the gas and the temperature drops. U = q + w U = w (since q = 0) In this case, the change in internal energy of the gas equals the work done by the gas. Figure 2: Reversible isothermal and adiabatic expansions From the P-V curves in figure 2, we see that when a gas expands adiabatically to a larger volume and lower pressure, the volume is smaller than it would be after an isothermal expansion to the same pressure. The energy for doing work in adiabatic expansion of a gas comes from self-cooling of the gas. Consider one mol of an ideal gas that expands reversibly and adiabatically. For an adiabatic process: 1 U = w or dU= dw =-pdV We saw earlier that dU Cv dT Therefore Cv dT PdV Now from P = nRT/V We get: Cv (i.e., ideal gas condition) dT dV R T V Which on integration: T2 V 2 dT dV gives: Cv R T V T1 V1 T V Cv ln 2 R ln 1 T1 V2 T2 T1 CV V 1 V2 T2 V1 T1 V2 where R CV R V T or 2 1 T1 V2 1 for an adiabatic expansion R C P CV 1 CV CV Since for one mol of an ideal gas, PV = RT PV T R V T PV then 2 2 2 1 T1 P1V1 V2 1 which gives P2V2 P1V1 Or PV constant Compare for adiabatic process PV = constant for isothermal process 2 Note: The student should also be able to show that for adiabatic process T2 P2 T1 P1 1 T or 2 T1 CP R P2 P1 Work Done on Ideal Gas along a Reversible Path V2 Work, w PdV V1 PV constant Since P (= b) for adiabatic expansion, therefore V b b b V21 V11 and w dV V 1 V V 2 1 gives: w P2V2 P1V1 1 w nRT2 T1 1 w nCV T nCV T2 T1 Summary Table: Work done, internal energy change and enthalpy change of an ideal monatomic gas due to different P-V-T processes. Constant Pressure Constant Volume Isothermal Adiabatic Variable Work done Molar Heat Capacity, Cm (J mol-1K-1) nCv(T2 – T1) Cp = (5/2)R Internal Energy change, ∆U = q + w Enthalpy, Cv = (3/2)R nCv(T2 – T1) nCp(T2 – T1) or or nCv(T2 – T1) nCp(T2 – T1) 3 Examples 1. When a perfect monatomic gas is allowed to expand adiabatically from 22.4 L at 1 atm and 273 K to a volume of 44.8 L, the pressure drops to 0.32 atm. Confirm this pressure and calculate the final temperature and the work done. Answers: T2 = 172 K and w = -1261 J. 2. A 2-mol sample of N2 gas at STP is expanded reversibly and adiabatically to a pressure of 0.2 atm. (i) Which one(s) of the quantities q, w, ∆U and ∆H is(are) zero for that process? (ii) Calculate the values of each of the non-zero quantities in (i) if any. 4