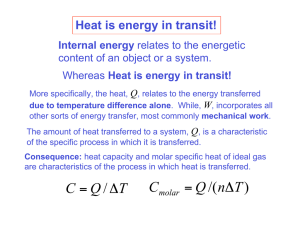
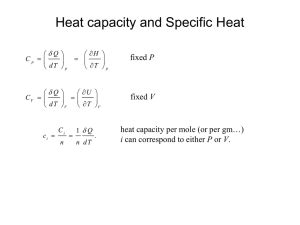
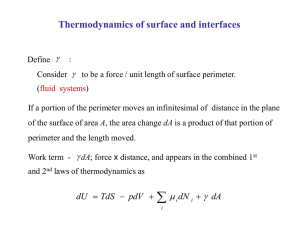
What is thermodynamics
advertisement
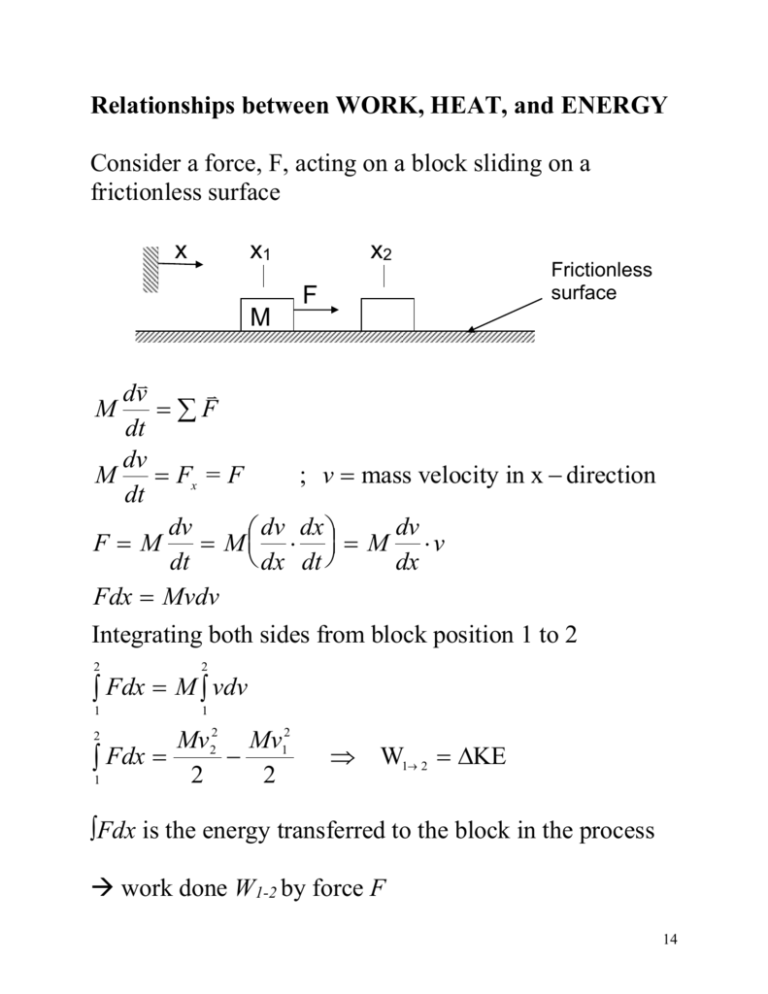
Relationships between WORK, HEAT, and ENERGY Consider a force, F, acting on a block sliding on a frictionless surface x x1 M x2 F Frictionless surface dv M F dt dv M Fx = F ; v mass velocity in x direction dt dv dv dv dx FM M M v dx dt dt dx Fdx Mvdv Integrating both sides from block position 1 to 2 2 2 1 1 Fdx M vdv Mv22 Mv12 Fdx 2 2 1 2 W1 2 KE ∫Fdx is the energy transferred to the block in the process work done W1-2 by force F 14 Consider an object falling in a gravitational field m y F=mg h1 h2 2 F dy (mg )dy mg (h1 h2 ) 2 W1 2 1 1 Gravity has the potential to do work and the quantity mgh is therefore called the potential energy Work done by gravity results in a drop in potential energy of the object since W1 2 KE (see previous example) mv22 mv12 mg (h1 h2 ) 2 2 PE KE Mass PE is converted to KE via the work done by gravity 15 Energy Transfer by Work In general, work done is evaluated by W1 2 F ds 2 1 Work is a means of transferring energy, it does not refer to what is being transferred or stored within the system. The value of W1 2 depends on the details of the interaction taking place between the system and the surroundings during a process, e.g., F(s), and not just the initial and final state By definition a state property is evaluated at a specific time and is independent of the process energy is a property of the system work is not a property of the system 16 The differential of a property is “exact” since it is independent of details of the process, e.g., 2 dE E 2 E1 1 Differential of work is “inexact,” the following integral can’t be evaluated without knowing details of the process 2 W W 2 not W W2 W1 1 1 The work done over a period of time is: 2 2 ds W F ds F dt F v dt dt 1 1 1 2 where v is velocity The rate of energy transfer by work is called power and is denoted by W . In general, W F v 17 Expansion and Compression Work Consider the expansion of the gas in a piston-cylinder assembly ( Pp is average pressure on piston face) Pp Pp x x1 x2 Piston Area A 2 2 F ds ( Pp A)dx Pp dV 2 W1 2 1 1 1 For a slow or quasi-equilibrium process all the states through which the system passes are considered equilibrium states and thus the intensive properties, i.e., pressure, are uniform throughout the system PP Pgas , so V2 W1 2 Pgas dV V1 18 Graphical Interpretation: State 1 P1 δW=PdV Pressure Process path State 2 P2 V1 V2 dV x1 W PdV 2 x2 shaded area V2 W1 2 W PdV 1 Volume total area under curve V1 19 Consider two processes with the same start and end state P1 State 1 Path 1 Path 2 State 2 P2 V1 V2 Since the area under each curve is different the amount of work done for each path is different. V2 V2 V1 V1 ( PdV ) path 1 ( PdV ) path 2 Work done depends on the path taken and not just the value of the end states. Work is not a property! 20 Polytropic Compression and Expansion The pressure-volume relationship can be described by PVn= constant c n= constant The work done is: V2 W1 2 V2 V2 c PdV ( n )dV (cV n )dV V1 V1 V V1 V2 V1 V 1 n V2 c c V1 1 n 1 n 1 n 1 n n n but c=PV PV 1 1 2 2 W1 2 n 1 n 1 n 1 n PV PV V V V 2 2 2 2 1 n 2 1 PV 2 2 1 n 1 n PV PV 1 1 W1 2 2 2 1 n n 1 21 For n=1 V2 c PdV dV clnV V1 V1 V1 V V2 W1 2 P = c/V V2 V2 clnV2 lnV1 c ln V1 V W1 2 P1V1 ln 2 V1 n=1 Special case: For n = 0 V2 P = c constant pressure process W1 2 PdV P(V2 V1 ) n0 V1 22 Spring Potential Energy F = x F – spring force = kx k – spring constant (N/m) x – displacement from relaxed position 2 kx 2 x2 W1 2 F ds ( kx )dx 2 1 1 x1 1 k ( x22 x12 ) 2 2 Spring PE 1 2 kx 2 The spring potential energy can be grouped in with gravitational potential energy. 23 Other forms of Energy In engineering, the change in total energy of a system is considered to be made up of macroscopic contributions such as changes in KE and gravitational PE of the system as a whole relative to an external coordinate frame and Internal Energy, U. E2- E1= (KE2- KE1) + (PE2- PE1) + (U2- U1) Consider the vigorous stirring of a fluid in a well insulated tank Well insulated Fluid Electric motor system W Energy is transferred into the system via work by the paddle wheel, results in an increase in the system energy. E2- E1= (KE2- KE1) + (PE2- PE1) + (U2- U1)= W This transferred energy does not increase the KE or PE of the system. 24 The change in system energy can be accounted for in terms of internal energy of the fluid. Changes in internal energy for solids, liquids, and gases are evaluated using empirical data, e.g. U = f(T) Microscopic Interpretation of Internal Energy Energy is attributed to the motions and configuration of the individual molecules, atoms and subatomic particles making up the matter in the system. Energy on molecular level associated with: - Translation - Rotation - Vibration - Molecular bonds Energy on atomic level: - Electron orbital states - Nuclear spin - Nuclear binding 25 Conservation of Energy for Closed System A closed system can interact with its surroundings via work as well as thermally Energy can be transferred between the system and the surroundings by thermal (heat) interactions A process that involves work interactions but does not involve thermal interactions is called an adiabatic process A process that involves thermal interactions is called a nonadiabatic process It has been shown experimentally that the net work done by, or on, a closed system undergoing an adiabatic process depends solely on the end states and not on the details of the process. E2 – E1 = -Wad Sign convention for energy transfer by work: Work done by the system is positive Work done on the system is negative 26 For a quasi-equilibrium adiabatic gas compression or expansion process the value of the polytropic exponent n is fixed (n =1.4 for air) and thus the area under the curve (work done) depends only on the end states P1 1 Adiabatic path PV1.4 = const (air) 2 P2 V1 V2 Consider an adiabatic process and nonadiabatic process between the same two end states 1 and 2 P1 1 Adiabatic (only work) PV1.4 = const. Nonadiabatic (work and heat) PVn = const. 2 P2 V1 V2 27 Since the area under the two curves is different the work done for each path is different, so Wad Wnonad Since the end states for both processes are the same the system would experience exactly the same energy change in each of the processes, so (E2 – E1)ad = (E2 – E1)nonad = E2 – E1 We know the energy change for the adiabatic process is E2 – E1 = -Wad But since Wad Wnonad we can infer that E2 – E1 -Wnonad Since energy must be conserved the net energy transferred to the system in both processes must be the same. It follows that the heat interaction in the nonadiabatic process must involve energy transfer. The amount of energy transferred to the closed system by heat is Q E2 – E1 = -Wnonad + Q The First Law of Thermodynamics states: E2 – E1 = Q - W 28 Energy Transfer by Heat The quantity Q in the First Law accounts for any energy transferred to a closed system during a process by means other than by work. Such energy transfer Q is induced only as a result of a temperature difference between the system and the surroundings and occurring in the direction of decreasing temperature, e.g. heat transfer: conduction, convection, radiation Sign convention for energy transfer by heat: Heat transfer to the system is positive Heat transfer from the system is negative Consider the immersion of a lump of hot metal initially at Tm into a colder fluid at Tf Tm Tf Tm > Tf Tf Q TmTf 29 Because the metal is at a higher temperature than the fluid energy is transferred from the metal to the fluid, Q is negative. Since there is no work done and the change in KE and PE is negligible, the amount of heat transferred from the metal to the fluid is equal to the decrease in the metal internal energy, U KE PE Q W Q = U1 – U 2 U2 - U1 = (-Q) or Just like work, heat is not a property and the amount of energy transfer depends on the process details, therefore 2 Q1 2 Q 1 The rate of heat transfer is denoted by Q and the total energy transferred via heat over a period of time is 2 Q1 2 Qdt 1 30