PowerPoint
advertisement
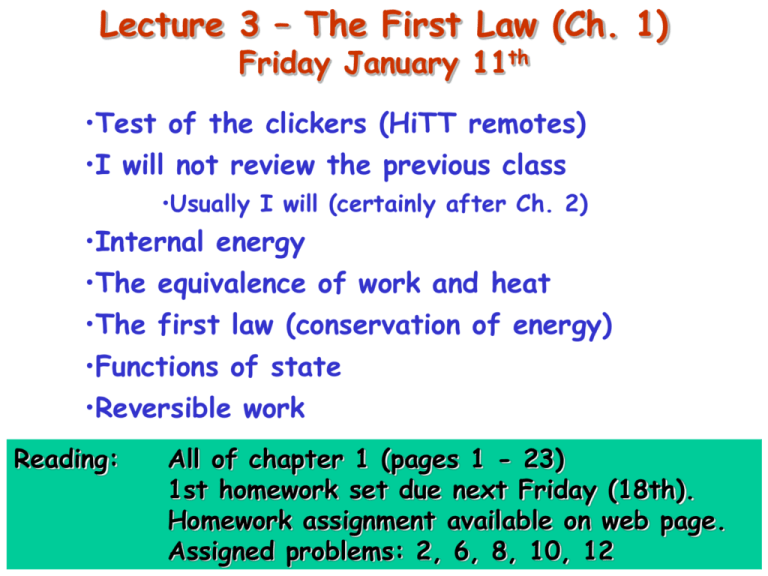
Lecture 3 – The First Law (Ch. 1) Friday January 11th •Test of the clickers (HiTT remotes) •I will not review the previous class •Usually I will (certainly after Ch. 2) •Internal energy •The equivalence of work and heat •The first law (conservation of energy) •Functions of state •Reversible work Reading: All of chapter 1 (pages 1 - 23) 1st homework set due next Friday (18th). Homework assignment available on web page. Assigned problems: 2, 6, 8, 10, 12 Functions of state: internal energy U Adiabatic Work = -DUgrav W = -(-mgh) = mgh Joule’s paddle wheel experiment Measured as a change in temperature, q Gravitational energy is lost. 1st law is about conservation of energy. This energy goes into thermal (‘internal’) energy associated with the fluid. Functions of state: internal energy U Adiabatic DUfluid = W = mgh !!!!!!!!!!!!!!!!!!!!!!! Joule’s paddle wheel experiment Measured as a change in temperature, q Gravitational energy is lost. 1st law is about conservation of energy. This energy goes into thermal (‘internal’) energy associated with the fluid. Functions of state: internal energy U Stirring Adiabatic Rise in q (temperature) DU = W = torque × angular displacement = t df Functions of state: internal energy U Electrical work Adiabatic Rise in q (temperature) i R DU = W = 2 i R Functions of state: internal energy U Reversible work Force, F Adiabatic Rise in q (temperature) DU = W = Force × distance = -P DV Equivalence of work and heat Heat, Q Adiabatic Same rise in q (temperature) DU = Q The First Law of Thermodynamics These ideas lead to the first law of thermodynamics (a fundamental postulate): DU = Q + W or dU = đQ + đW “The change in internal energy of a system is equal to the heat supplied plus the work done on the system. Energy is conserved if the heat is taken into account.” Note that đQ and đW are not functions of state. However, dU is, i.e. the correct combination of đQ and đW which, by themselves are not functions of state, lead to the differential internal energy, dU, which is a function of state. How to know if quantity is a function of state U1 W PdV area under curve Significant heat flows in U2 How can U be state function, but not W? Heat is involved (not adiabatic). DU đQ + đW How to know if quantity is a function of state There is a mathematical basis..... Consider the function F = f(x,y): f f dF dx + dy x y y x z dS y dF x dr How to know if quantity is a function of state There is a mathematical basis..... Consider the function F = f(x,y): f f dF dx + dy x y y x In general, F is a state function if the differential dF is ‘exact’. dF (= Adx + Bdy) is exact if: 1. 2. A B y x See also: •Appendix E •PHY3513 notes •Appendix A in Carter book dF 0 dF is independent of path b 3. a •In thermodynamics, all state variables are by definition exact. However, differential work and heat are not. How to know if quantity is a function of state Differentials satisfying the following condition are said to be ‘exact’: dF 0 This condition also guarantees that any integration of dF will not depend on the path of integration, i.e. only the limits of integration matter. This is by no means true for any function! If integration does depend on path, then the differential is said to be ‘inexact’, i.e. it cannot be integrated unless a path is also specified. An example is the following: đF = ydx - xdy. Note: is a differential đF is inexact, this implies that it cannot be integrated to yield a function F.