Heat is energy in transit! Internal energy Q
advertisement

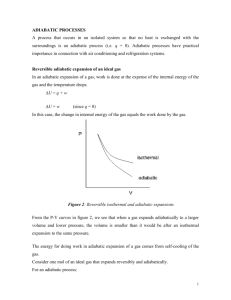
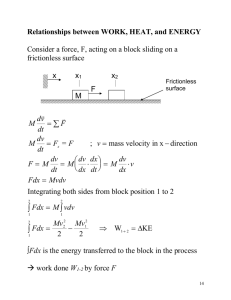
Heat is energy in transit! Internal energy relates to the energetic content of an object or a system. Whereas Heat is energy in transit! More specifically, the heat, Q, relates to the energy transferred due to temperature difference alone. While, W, incorporates all other sorts of energy transfer, most commonly mechanical work. The amount of heat transferred to a system, Q, is a characteristic of the specific process in which it is transferred. Consequence: heat capacity and molar specific heat of ideal gas are characteristics of the process in which heat is transferred. C = Q / !T Cmolar = Q /( n!T ) Isothermal process – T = const Isotherm "U = Q ! W "U = 0 ! Q =W What is molar specific heat of ideal gas in an isothermal process? CT = Q /( n!T ) We have got: Q > 0, #T = 0 " CT = ! Constant volume (isochoric) process – V = const. "U = Q ! W W =0 Q = !U By definition of the molar specific heat at constant volume, Cv , Q = nCv !T n – number of moles of the gas. Therefore: 1 !U Cv = n !T !U = nCv !T Measuring Cv we learn about internal energy of the gas as a function of temperature! From the 1st law and equation for Cv: Q = !U + W = = nCv !T + P!V = P V = n(Cv + R)!T Molar specific heat at constant pressure (definition): C p = Cv + R Q = nC p !T Why is specific heat at constant pressure higher than at constant volume? Heat capacity is defined as, C=Q/ΔT. It depends on heat transferred to the system, NOT on the change in the internal energy. Therefore, heat capacity is a function of the process and is different for different processes. More specifically, if the volume is kept constant, the entire heat is used to increase the system’s internal energy. If volume increases, the heat is used in two ways: to increase the internal energy and to perform work, Q = ΔU+W. Therefore, more heat is required per 1° temperature increase. 4 processes between two isotherms. How do you order them in terms of heat transferred to the gas? Things to remember: U depends on T only; W = ! PdV Q = !U + W Thermodynamic processes… "U = Q ! W PV = nRT W = ! PdV What is the heat transferred to the gas in the process? Q = !U + W W = ! PdV !U = nCv !T PV nT = R P2V2 ! P1V1 n"T = R P2V2 " P1V1 Q = Cv + ! PdV R Adiabatic processes – no heat transfer (thermal insulation). Q=0 PV = nRT "U = !W W = ! PdV Adiabatic processes – no heat transfer (thermal insulation). Q=0 "U = !W Positive work, W, is done by the expanding gas at expense of reduction of its internal energy… Since there is no heat supplied from the outside to replenish the gas energy, the temperature decreases. BTW, what is the molar specific heat of a gas in an adiabatic process? Adiabatic processes… Blue arrow – the gas is expanding, does a positive work and is cooled down (“goes to lower isotherms”)… Red arrow – the gas is contracting, driven by some external forces. It does negative work and heats up (“goes to higher isotherms”). An isothermal process is described by P1V1 = P2V2 An adiabatic process is described by ! 1 1 PV = P2V2 ! nRT const P= = V V const P= ! V γ = Cp/Cv is a constant, such as 1< γ < 2 It is different for different gases! Fire Syringe: A small wad of cotton bursts into flames when the air in a narrow tube is rapidly compressed. How come, the two equations may work for the same ideal gas? ! 1 1 P1V1 = P2V2 PV = P2V2 ! Are they compatible with the ideal gas law? PV = nRT Yes, they are both compatible! Because the ideal gas law has 3 variables, and P as a function of V in a particular process depends on what happens to the temperature! Isothermal T = const Adiabatic TV = const const T = " !1 V " !1 Cyclic processes What is so special about them? The system returns to the same point (state) P What can we say about change ΔU, Q and W ? Since the system returns to the same state and V U is a function of state only, !U Therefore by the 1st law we have A B =0 "U = Q ! W W =Q So, the system gets a net heat, Q, from the outside and does an equal amount of work, W. Or the other way around! Both Q and W may be negative! What simple thermodynamic process are cyclic processes similar to? In what are they different? Cyclic processes "U = Q ! W Since ΔU = 0, all we have to do is calculating W. How do we do that? P V Net work In the simplest case we can divide the process into two parts: expansion A → B, with positive work done; contraction B → A with negative work done. Question: the gas goes from the state i to the state f . What is the minimal work that gas can do in the process? What is the pressure is not allowed to drop below Pf ? Any other suggestions? 1 2 We can start at i , got to 1, make, say 10 circles, go to 2 and only then continue to f. 10 loops 1 2 With the 10 loops, and negative net work in each loop, the total work is certain to be negative!