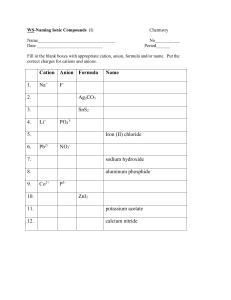
ANALYZE Denjiro Glenda (a new kid in class) Debra (a long-term friend of Denjiro) Denjiro Lecture 1. NAMING IONIC BINARY COMPOUNDS that contains cations formed by transition metals USING THE CLASSICAL SYSTEM 2. FORMULA WRITING Naming binary ionic compounds that contains cations of transition metals using the classical system . Oxygen Anion w/ ) Examples: FeO (Iron cation w/ and 2+ 2- Fe2O3 (Iron cation w/3+ and Oxygen Anion w/2- ) STEPS Step 1: Write the name of the cation • Write first the root word of the metal that formed the cations. • Add the proper suffixes: for lesser charge cation, use the suffix –ous and for cation with greater charge use the suffix –ic. Step 2: Write the name of the anion Work on these examples: CuCl: Copper cation with 1+ (Cu+) and Cl Anion with1- (Cl-) (root word for cupper is Cupr- and the root word for is Chlorine is chlor-) 1. 2. CuO: copper cation with 2+ (Cu2+) and Oxygen anion with 2- (O2-) (root word for copper is Cupr- and the root word for oxygen is Ox-) Other examples you can work on: 3. FeI2: Iron cation with 2+ (Fe2+) and Iodine anion with 1- (I-) (root word for Iron is Ferr- and Iodine is iod-) 4. FeBr3: (Iron cation with 3+ (Fe3+) and Bromine anion with1- (Br-) (root word for Iron is Ferr- and the root word for bromine is brom-) Answers: Cuprous Chloride Cupric Oxide Ferrous Iodide Ferric Bromide Formula Writing Examples: Examples: Ferrous Oxide (Iron cation w/2+ and Oxygen Anion w/2- ) Ferric Oxide (Iron cation w/3+ and Oxygen Anion w/2- ) Steps in formula writing. Step1: Write first the symbol of the metal that formed the cation and followed by the symbol of the nonmetal that formed the anion. Step 2: Write their respective charges Step 3: Use the crisscross method: cancel the charges sign, and the numerical value of the charge of an ion will become the subscript of the other ion. To write formula for ionic compounds consisting of ions that have the same charge numerical value, follow this Rule: Write the symbol of the metal that formed the cation and followed by writing the symbol of the non-metal that formed the anion. Example: The formula of Ferrous oxide that is composed of Fe2+ and O2- is written as? FeO Do this! . Write the formula of the following examples: Do this on the board! 1. Cuprous chloride: Cu+ and Cl2. Cupric chloride: Cu3+ and Cl3. Auric bromide: Au3+ and Br5. Ferrous Sulfide: Fe2+ and S2- Answers: CuCl CuCl3 AuBr3 FeS PRACTICE The following steps of the activity will be followed; Step 1: The teacher will flash two cards: a cation and an anion Step 2: After the teacher flashed the cards, write the formula and name of the ionic compound that can be formed by combining the cation and anion. Write your answer on a worksheet. 20 seconds are given to everyone to write their answer. Read out loud and then analyze! During his work, Dr Herbert asked his new assistant, Cecil to get the bottle, labeled with Hg2Cl2: The compound Hg2Cl2 is a purgative. After Cecil heard the order of Dr. Herbert, he immediately went to the drug storeroom, and inside the storeroom, he found a bottle labeled as HgCl2: Mercuric chloride, he took the bottle without hesitation. When Dr. Herbert got the bottle, he instantly noticed something wrong. What did Dr Herbert, noticed? Is it important to know exactly the chemical name and the formula of a compound in our daily life? SEATWORK Homework