Key 2
advertisement

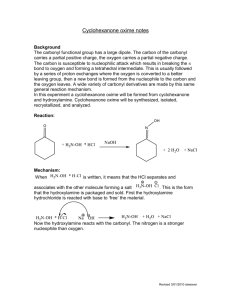
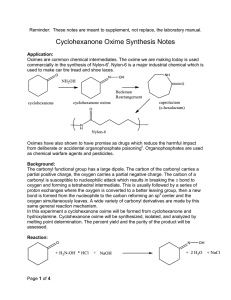
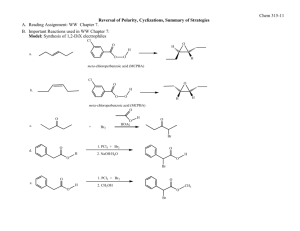
The 3o alcohol is a retron for nucleophilic addition to 2-methylcyclohexanone. All the synthetic plans given below are based on the nucleophilic addition to 2-methylcyclohexanone. Retrosynthesis (1-5) is based on the use of acetylide as acyl anion equivalent.1, 2 Attack of the nucleophile on cyclohexanone should be a stereoselective reaction. The nucleophile should attack from equatorial position to give the desired relative stereochemistry. Hydrolysis of product will give ketone 4.3, 4 Michael addition of 2 with 3 will give the target molecule.5 This will require protection of alcohol in 4 prior to addition.5 4 has been prepared by reduction of beta diketone 7, but was discarded as yield was only 40 %.6 Scheme-1 O O (12) (10) Protection O Selective protection at both ends O S + (11) O S O O + O O + O (9) Nucleophilic addition to cyclohexanone, deprotection Nucleophilic addition to cyclohexanone (umpolung) ,deprotection (4) Protection of alcohol O O Me3SiO O O HO (3) deprotection (1) (2) Michaels addition to alpha beta unsaturated aldehyde, deprotection (8) O OH Reduction of beta diketone (7) CHO + Target Molecule O (5) Nucleophilic addition of cyclohexanone O HO O O HO O + (6) CHO (3) Scheme-1 Retrosynthesis (1-10) and (1-12) are shorter routes. Both of these eliminate the Michael addition step of retrosynthesis (1-5). Retrosynthesis (1-10) was discarded as it had a problematic step of selective protection at both the ends of 10 to produce 9. Route (1-12) looks good. 11 can be synthesized by commercially available 12 in one step in good yield.7 Relative stereochemistry will follow the same arguments given in route (1-5). This route is taken in the direction of forward synthesis, (Scheme-2) as it is shorter. The attacking nucleophile is bulkier and hence it should give better stereoselectivity. Proposed synthesis: Alkyne (11) is a known material, prepared from (12) which is a commercially available material. Specific example of this kind of protection of carbonyl group is found and outlined in scheme 3.7 Use of acetylide as acyl anion is a well known reaction. Hydrolysis of alkyne gives ketone. (Scheme-4).1, 2 O O O HO ethylene glycol, H O 4 O O heptane, reflux 3 O 1.1. BuLi, THF 2. Hg2 , H3O O aq AcOH HO O Scheme-2 O ethylene glycol, H O heptane, reflux O Scheme-3 (a) CH CLi (b) H3O+ , Hg2+ 85% OR O R1 O R1 R2 O NaC CH R2 in liq. NH3 Scheme-4 R2 OH C CH R3 Considering the issue of stereoselectivity, attack of the bulkier nucleophile on cyclohexanone should be a stereoselective reaction. The nucleophile should attack from equatorial position; it should give the desired relative stereochemistry. O OH R R Scheme-5 The closet precedents found from the literature are outlined below.4 OH O MgBr THF, OoC References: 1. Milas, N. A.; MacDonald, N. S.; Black, D. M. J. Am. Chem. Soc. 1948, 70, 1829. 2. Linden, E.; Palomo, C.; González, A.; García, M. J.; Landa,C.; Oiarbide, M.; Rodríguez, S. Angew Chem. Int. Ed. 1998, 37, 180. 3. Tarr, C. J.; Johnson, S. J. Org. Lett., 2009, 11, 3870. 4. Cl e´ment, R.; Grise´, C. M.; Barriault, L. Chem. Commun. 2008, 3004. 5. Clayton H. Tetrahedron Lett. 1986, 27, 6169. 6. Kariv, E.; Wenker, E.; Chem. Commun. 1965, 22, 570. 7. Wuts, G. M.; et. al. Org. Process Res. Dev. 2009, 13, 331. total score 22 / 25 Criteria: 1) (5 pts.) Does the student have an appreciation for the molecular complexity of the structure and does the solution effective solve these problems. 5/5: You did well in this criterion. 2) (10 pts.) Are the precedents given to support the synthetic proposal well-documented? Are the precedents close. 9/10 You did well here too. 3) (5 pts.) Are the commercial sources cited? Does the student tell me where they are going to get the starting materials? 3/5 You chased your bits and pieces down to commercial sources but you did not state where you found the molecules. Quoting a price and source was expected. I am going to be lenient for the first assignment. 4) (5 pts.) Are at least two COMPETITIVE retrosynthetic schemes offered and a logical argument given about why one of these designs is better? 5/5 You did very well here. The retrosynthetic analyses that you chose to develop into a forward synthesis had documentation problems. I think if you look you probably would have found precedent for the regiochemistry of hydration of the triple bond. . You will get better at this with a little practice. In general you did a great job.