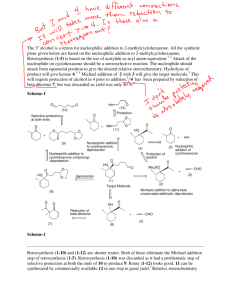
Answers
advertisement

Nucleophilic Addition with strong nucleophiles Major concepts Nucleophilic addition is the major mechanism of electrophiles such as aldehydes and ketones When a strong nucleophile attacks the partially positive carbon of a carbonyl, an sp3 hybridized carbon atom results Vocabulary Nucleophilic addition Carbonyl Ketone, aldehyde Enolate LDA Students should be able to: Draw a mechanism for a nucleophilic addition of a strong nucleophile to a ketone or aldehyde Predict the products of nulceophilic addition reactions, particularly directed aldol Given an aldol product, determine the structure of the nucleophile and electrophile Daily Problems 1. Which of these molecules would be capable of undergoing a nucleophilic addition? Which aren’t? What characteristic separates them? O A. O O B. HO C. O O O D. H E. . Cl F. O H2N . . . A. Cannot undergo nucleophilic addition because the H on the O is acidic therefore the nucleophile would act as a base first because acid/base reactions are very fast. B. Cannot undergo a nucleophilic addition because –OCH3 is a good leaving group. C. Can undergo a nucleophilic addition because there is no leaving group. D. Can undergo nucleophilic addition because there is no leaving group. E. Cannot undergo nucleophilic addition because –Cl is a good leaving group. F. Cannot undergo nucleophilic addition because –NH2 is a decent leaving group. 2. Propose a nucleophilic addition mechanism for this reaction. (Hint: Start with the catalyst.) 3. Propose a mechanism for this nucleophilic addition. Notice that it has two steps, because the strong nucleophile would react with acid if it were present initially. 4. In this three step reaction, the strong nucleophile was made first, then the nucleophilic addition, then the acid quench. Fill in the products of each step. 5. Use question 4 as a model for predicting the product of this reaction: 6. Identify the nucleophilic center and electrophilic center in this reaction, and then draw the product. nuc E+ Cumulative Problems 7. What are the similarities and difference between a nucleophilic addition reaction and an electrophilic addition reaction? Explain and give an example of each. How can you recognize they are different based on their starting materials? Nucleophilic additions and electrophilic additions are similar in that they both are reactions of a nucleophile and electrophile with one of the two being a pi bond. The differences lie in that the nucleophile is adding into an electrophilic pi bond in nucleophilic addtions and the pi bond is acting as a nucleophile in electrophilic additions. Example of a nucleophilic addition: Example of an electrophilic addition: 8. What are the similarities and difference between a nucleophilic addition reaction and a nucleophilic substitution reaction? Explain and give an example of each. How can you recognize that they are different based on their starting materials? A nucleophilic addition reaction is the reaction of a strong nucleophile with an electrophile without a leaving group (i.e. an aldehyde or ketone). A nucleophilic substitution is the reaction a strong nucleophile with a molecule with a good leaving group that results in the leaving group being replaced with the nucleophile. They are similar in the attack of the nucleophile to an electrophile. They differ in that the substitution results in the loss of a leaving group where the addition does not. Example of a nucleophilic addition: Example of a nucleophilic substitution: 9. These two different reactions have the same nucleophile but give different products. Draw the products, and explain why the products and mechanisms of reaction are different. The products are different because the first is a substitution where –CN acts as the nucleophile to displace the Br leaving group and the second is an addition of –CN into an electrophilic pi bond making an OH instead of causing a leaving group to leave. 10. A conjugated carbonyl such as in but-2-enal has two centers that are open to attack by a nucleophile. Draw but-2-enal and explain why there are two electrophilic centers. Due to resonance structures, there is a partially positive or electrophilic carbon in two places on the molecule. Extension problem: 11. In this reaction, one equivalent of the aldehyde is deprotonated, and acts as the nucleophile. The second equivalent of aldehyde is the electrophile. Give a mechanism for the first arrow. The second reaction is a basic elimination exactly like the one you learned. Predict the product of this reaction. (Hint: Consider the basic elimination of -hydroxyaldehydes.)