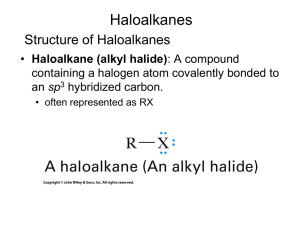fall_exp_extra6
advertisement

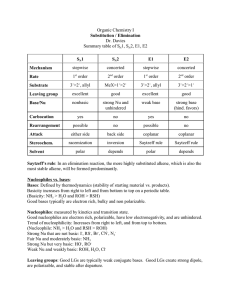
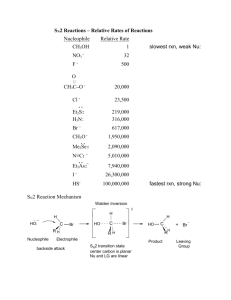
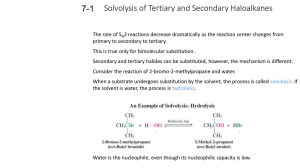
Fall Organic Chemistry Experiment #7 SN1 and SN2 reactions Suggested Reading: Jones Chapter 7 Introduction: This experiment is designed to illustrate some of the concepts introduced in Chapters 7 and 8 of your text. These two chapters include two of the major reaction categories in organic chemistry, substitution reactions and elimination reactions. We will employ these two experiments as a means of investigating some of the critical features of substitution reactions. We will be especially interested in learning about some of the basic criteria used to describe organic reactions (e.g. solvent effects, nucleophilicity, basicity, thermodynamics, kinetics, catalysis, etc.) and how these criteria may influence the course of a given reaction. One thing that we can consider is how to predict the major reaction pathway (S N2, SN1, E2, E1) based upon the given reaction conditions. That is, how do we know which reaction mechanism provides the correct description of the actual reaction pathway? Is it a concerted SN2 reaction or the stepwise SN1 with a carbocation intermediate? Well, the "correct mechanism" is the one that has been the most widely accepted in the primary chemical literature and accurately describes experimental results. Many times we can make a reasonable prediction based upon a complete analysis of the substrate and reagents involved. If you notice, the starting material contains a leaving group attached to a primary -carbon. Therefore, a good starting point for predicting the outcome of a reaction is the evaluation of the specific variables that can selectively influence which reaction (SN2, SN1, E2, or E1) will predominate. Definitely spend some time thinking about all of the variables responsible for optimizing each reaction category. It will certainly help to read the material in Chapter 7. Some of the more important variables include: (a) Structure of the substrate (e.g. primary, secondary, or tertiary alkyl halide) (b) Type of solvent (c) Strength of the nucleophile (d) Basicity of the nucleophile Today's experiment focuses on two major thematic variables: (a) nucleophilicty -- how the type of nucleophile influences the course of a reaction and (b) kinetics (rate-determining step) -how we can use kinetic data to verify the mechanism of reaction. The first theme focuses on the nature of reaction conditions in influencing the course of reaction and the second is purely a look at how mechanisms are verified. In either case, it is the actual experimental findings that indicate something about what is occurring at the molecular level in the reaction. Part 1 (Competing Nucleophiles) "Not all nucleophiles are created equal". Some nucleophiles (like the cyanide ion) are quite strong and others (like methanol) are weaker -- see Table 7.3 in your text or the Table on page 101 of the purple book. It should be noted that nucleophilic strength is not really this straightforward. In fact, the actual strength of the nucleophile is dependent upon the reaction solvent. The general trend is that the nucleophilicity of anions is enhanced in polar, aprotic solvents rather than polar, protic ones. In other words, the nucleophile is more available in the polar, aprotic solvent because of its decreased hydrogen bonding interaction with the solvent. In this experiment, we will be using a polar protic solvent. This piece of information is quite important because in polar, protic solvents, the nucleophilicity of anions is exactly the opposite of the basicity (reversed). Therefore, the more basic the anion, the less nucleophilic it will be. If the strength of one nucleophile is decreased and everything else remains equal, then that nucleophile will not be able to compete effectively. Ineffective competition leads to a lower percentage of the product distribution. If we can measure the actual distribution of products, then we can determine whether one nucleophile is indeed stronger than the other. In this experiment, our goal is to compare the relative nucleophilicities of the chloride ion and the bromide ion in two different reactions. In the first reaction, chloride and bromide will compete for 1-butanol (most likely an SN2 reaction -- why?). In the second reaction, chloride and bromide will compete for 2-methyl-2-propanol (most likely an SN1 reaction -why?). Both nucleophiles are present simultaneously in the reaction mixture at equimolar concentrations. The goal in this experiment is for you to analyze the products of the two reactions by nuclear magnetic resonance spectroscopy (NMR) in order to determine the relative amounts of each product. In addition, you will determine which ion is the stronger nucleophile and for which of the two reactions this difference is important. Procedure This experiment has already been run and the NMR spectra have been obtained. They will be distributed to each of you during the lab period. Your job is simply to use the data collected to determine which product predominates in each reaction and why. You should also suggest how these results reflect the relative nucleophilicities of the two attacking nucleophiles. In addition, you should be able use this data to define the role of the nucleophile in both the S N1 and SN2 reactions. Part 2 (Solvent Effects in Nucleophilic Substitution Reactions) The information for the second part of today’s experiment can be found at the following web addresses: The experiment that we will be performing is: What Role, If Any, Does Solvent Play in Nucleophilic Aliphatic Substitution Reactions? Before lab you should read through the virtual pre-lab that can be found at: http://creegan.washcoll.edu/gilabs/solvolysis_rx_%20folder/solvolysis_rx_vpl.html You should also complete the 3 pre-lab questions (we will discuss at the beginning of the lab session) at the following site: (Be sure NOT to submit online!!!! – simply answer in lab notebook) http://creegan.washcoll.edu/gilabs/solvolysis_rx_%20folder/solvolysis_rx_prelabqs.html You might also check out the PowerPoint simulations (RBr simulation and RCl simulation) that illustrate in more detail the procedure for today’s experiment at: http://creegan.washcoll.edu/gilabs/ The team assignments for today’s experiment are as follows: Names Alkyl Halide Solvent System Brian and Victoria 2-chloro-2-methylpropane 2-propanol-water Mark and Matt 2-chloro-2-methylpropane acetone-water Lindsey and Lindsey 2-bromo-2-methylpropane 2-propanol-water Lynn and Cayleigh 2-bromo-2-methylpropane acetone-water Lauren, Jamie, and Kay 2-iodo-2-methylpropane 2-propanol-water Brittany and Greg 2-iodo-2-methylpropane acetone-water Lauren, Jamie, and Kay 1-chlorobutane 2-propanol-water Brittany and Greg 1-chlorobutane acetone-water Nick and Kevin 1-bromobutane 2-propanol-water Nick and Kevin 1-bromobutane acetone-water