Title: Unsymmetrical Substituted 2,5
advertisement

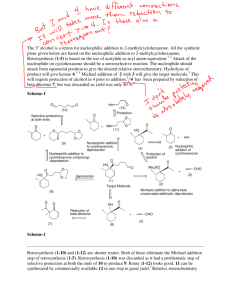
Title: Unsymmetrical Substituted 2,5-Diarylidene Cyclohexanone as anti-parasitic agents Zia ud Din1, Alef Dos Santos1, Edson Rodrigues-Filho1* 1 LaBioMMi, Departamento de Química, Universidade Federal de São Carlos, CP 676, 13.565- 905, São Carlos, SP, Brazil. Introduction: Unsymmetrical bis-aryl α, β-unsaturated cyclohexanone are the class of compounds having cyclohexanone attached to two aryl system at β-positions, structurally similar to curcuminoids. Direct synthesis of these unsymmetrical building blocks by Claisen-Schmidt condensations [1] was not possible. These moieties were synthesized via a two-steps reaction; the first step involved running a DIMCARB-catalyzed reaction of cyclohexanone with anisaldehyde to form monoarylidene cycloadduct [2], while the second step involved reacting B1 with various aldehyde under base-catalyzed conditions. DIMCARB can be recoverd by distillative dissociation-reassociation in vacuo or under an atmosphere of CO2. Bis-(arylmethylidene)-cycloalkanones and its analogues have been found to display great potential applications, such as anti-inflammatory, anti-tumor [4], antioxidant [5], antitubercular, cytotoxic, antifertility, and antifungal activities [12]. It has been proposed that the activity of this class of compound is related to their ability to act as Michael acceptors with nucleophilic biological macromolecules, especially nucleic acids and enzymes. Furthermore, researchers have proved that two aromatic regions of curcumin analogues might be important for potential protein-ligand binding and became a rational design of potential inhibitor (ligand) of protein targets. Chagas’ disease and Leishmaniasis are diseases caused by protozoan parasites. Chagas’ disease is found mainly in Latin America and about 7 to 8 million people are estimated to be infected worldwide. Leishmaniasis affects the poorest people on the planet, estimated 1.3 million new cases and 20 000 to 30 000 deaths occur annually in 98 countries. Among all leishmaniasis, the visceral form is most severe . In the absence of suitable medication, it has a mortality rate of almost 100% independent of the immunological position of the patient . The drugs available in the market are inadequate due to extreme toxicity, cost and ineffectiveness [20-21]. Pentavalent antimonials, which were developed in 1940s, are presenting high toxicity and low efficiency. Recently, more than 60% of VL patients in Bihar (India) are unresponsive to the antimonials. Pentamidine presents several side effects, including renal and hepatic toxicity, hypotension, pancreatitis, and cardiac abnormalities Amphotericin B and its formulations are quite active for VL, however, these are very costly, toxic, and have a longer half-life [24]. Until now, no specific vaccine is appropriate for human use Therefore, there is an urgent need for the development of new, effective, inexpensive, and harmless drugs for the cure of leishmaniasis. So study is needed to explore less toxic drugs to treat both diseases. Key words: DIMCARB, anti-parasitic agent, leishmania. Acknowledgment: Author are greatfull to TWAS-CNPq, FAPESP, CAPES for finantial assessment. References: [1] [a] L. Gall, E. Texier-Boullet, J. Hamelin, Simple Access to α, β Unsaturated Ketones by Acid-Catalyzed Solvent-Free Reactions, J. Synth. Commun. 29 (1999) 3651.[b] D.S. Noyce, W.A. Pryor, Carbonyl Reactions. Kinetics and Mechanism of the Acid-catalyzed Aldol Condensation of Benzaldehyde and Acetophenone. J. Am. Chem. Soc. 77 (1955) 1397-1401. (2) U.P. Kreher, A.E. Rosamilia, C.L. Raston, J.L. Scott, C.R. Strauss, Direct Preparation of Monoarylidene Derivatives of Aldehydes and Enolizable Ketones with DIMCARB, Org. Lett. 5(2003) 3107-3110. . [3] K.H Lee, F.H.A. Aziz , A. Syahida, F. Abas, K. Shaari, D.A. Israf, N.H. Lajis, Synthesis and biological evaluation of curcumin-like diarylpentanoid analogues for anti-inflammatory, antioxidant and anti-tyrosinase activities, Eur Jour of Med Chem. 44 (2009) 3195–3200. [4] S. Inayama, K. Mamoto, T. Shibata, T. Hirose, Structure and Antitumor Activity Relationship of 2-Arylidene-4-cyclopentene- 1,3-diones and 2-Arylideneindan- 1,3-diones, Jour Med of Med Chem. 19(1976) 433-436. [5] M.A. Simonyan, K. Dib, A.N. Pashkov, A.V. Simonyan, O.V. Myachina, O.V. Ostrovskii, Synthesis and antiradical and antioxidant activity of cycvalon and its analogs, Pharm. Chem. J. 41 (2007) 7-10.