NPBs11-7-Sum#1Revers..
advertisement

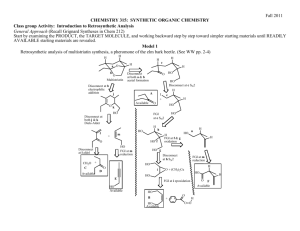
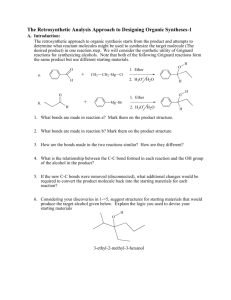
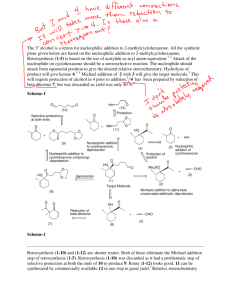
Reversal of Polarity, Cyclizations, Summary of Strategies A. Reading Assignment: WW Chapter 7. B. Important Reactions used in WW Chapter 7: Model: Synthesis of 1,2-DiX electrophiles Cl O H a. O O H O H meta-chloroperbenzoic acid (MCPBA) Cl O O H b. O O H H meta-chloroperbenzoic acid (MCPBA) O O c. + Br2 O HOAc H O Br d. 1. PCl3 + Br2 O H O O 2. NaOH/H2O H O Br e. 1. PCl3 + Br2 O H O O 2. CH3OH O Br CH3 Chem 315-11 2 Chem 315-11 Exploration: The products in reactions a-e are starting materials revealed in the 2-Group Di-X activity. Now we are exploring how they can be synthesized and the nature of their reactivity: 1. Identify the 1,2-DiX relationships in each product above. 2. Identify the potentially electrophilic site(s) in each product. 3. WW refers to these reagents as ones with “reversal of polarity”. Compare the reactivity of the carbon atoms identified in 2 in the products to the reactivity of the same carbon atom in the reactants of a-e and explain what the authors mean by “reversal of polarity”. Summary of Strategies for Retroanalysis This section is a review of the strategies we have been discovering so far this semester. Most is review, but the consideration of cyclizations has not been emphasized previously. (Compare these lists to the ones in WW Chapter 1, p. 4) WW illustrates some of the strategies with a discussion of the synthesis of Salbutamol. 1. Recognize the functional groups in the target molecule. 2. Disconnect by known reliable methods using FGI's if necessary to produce the best functional groups for disconnections. 3. Best disconnections to look for first are: a. Bonds joining an aromatic ring to the rest of the molecule. (Chapters 2&3) b. Any C-X disconnections especially: (1) Bonds next to carbonyl groups. (Acyl C-X -- Chapter 4) (2) 2-group C-X disconnections of 1,1-, 1,2- or 1,3- systems. (Chapter 6) (3) C-X disconnections of bonds within rings because cyclizations are favorable. (Chapter 7) Recall the syntheses of the compounds below froe the 2-Group Di-X activity. H O O O N H O 4. Repeat as necessary to reach available starting materials. 3 1. 2. 3. 4. Chem 315-11 Conversion of a Retrosynthesis to a Synthetic Scheme Write out the plan according to the retrosynthesis, adding relevant reagents and reaction conditions. Check that a rational order of events has been chosen. (See guidelines from Chapter 3) Check that Chemoselectivity is favorable (Chapter 5). Use protecting groups if necessary. (Chapter 9) Modify the planned synthesis to correct for problems detected in #'s 2 & 3 above. Application: In our first activity “Summary of Retrosynthetic Approach to Organic Synthesis” we explored a retrosynthetic analysis of multistriatin and converted one of the retrosynthetic branches into a synthesis. In chapter 1, WW discusses the retrosynthetic analysis of multistriatin but follows a branch that is somewhat different from the ones we used in the first activity. Also, in our first couple of classes, we had few tools to allow us to engage in the retroanalysis. So our application for this activity is to: 1. Write out the retrosynthetic analysis for multistriatin described in WW chapter 1, pp. 2 & 3, and identify the disconnections they used and other potential 2-Group-DiX disconnections they might have used. Then indicated how the “Summary of Strategies for Retroanalysis” were used or ignored in the retroanalysis. 4 Chem 315-11 2. Analyze the synthesis that they developed from the retroanalysis. (p. 4) Indicate how it deviated from the retroanalysis and how in does or does not illustrate the “Conversion of a Retrosynthesis to a Synthetic Scheme”.