HW4
advertisement
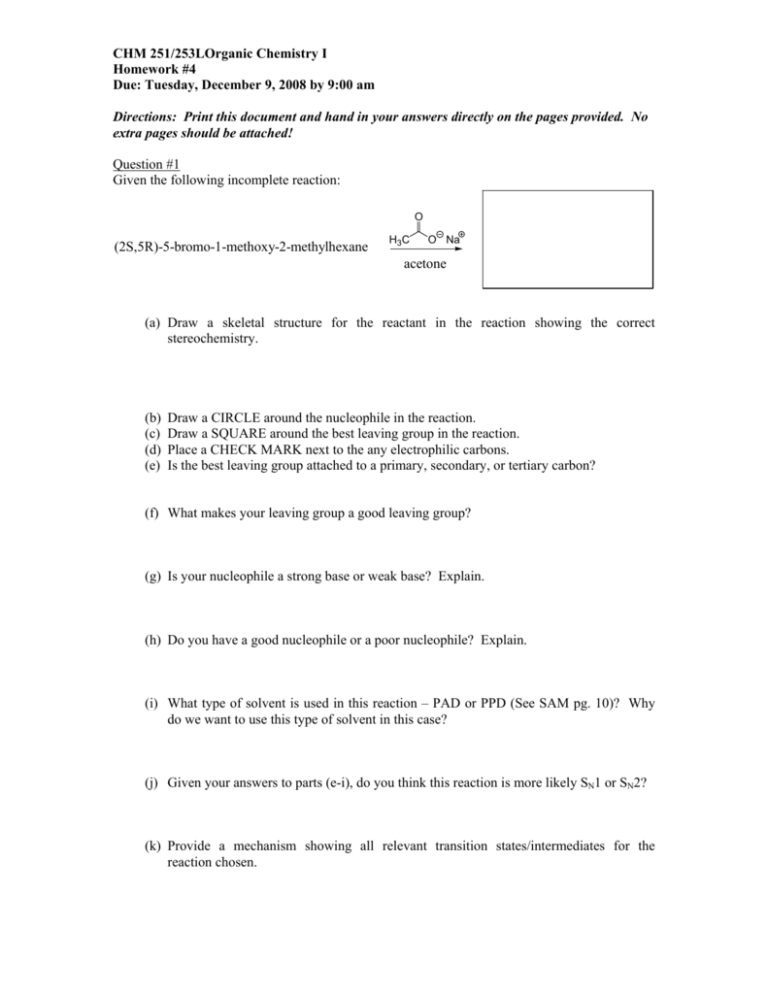
CHM 251/253LOrganic Chemistry I Homework #4 Due: Tuesday, December 9, 2008 by 9:00 am Directions: Print this document and hand in your answers directly on the pages provided. No extra pages should be attached! Question #1 Given the following incomplete reaction: O (2S,5R)-5-bromo-1-methoxy-2-methylhexane H3C O Na acetone (a) Draw a skeletal structure for the reactant in the reaction showing the correct stereochemistry. (b) (c) (d) (e) Draw a CIRCLE around the nucleophile in the reaction. Draw a SQUARE around the best leaving group in the reaction. Place a CHECK MARK next to the any electrophilic carbons. Is the best leaving group attached to a primary, secondary, or tertiary carbon? (f) What makes your leaving group a good leaving group? (g) Is your nucleophile a strong base or weak base? Explain. (h) Do you have a good nucleophile or a poor nucleophile? Explain. (i) What type of solvent is used in this reaction – PAD or PPD (See SAM pg. 10)? Why do we want to use this type of solvent in this case? (j) Given your answers to parts (e-i), do you think this reaction is more likely SN1 or SN2? (k) Provide a mechanism showing all relevant transition states/intermediates for the reaction chosen. (l) Provide skeletal structure(s) of the expected major product(s) in the box provided above. (m) How would the reaction change if you made the following alterations to the reaction conditions? 1. you change the nucleophile to (sodium) ethoxide and the solvent to ethanol 2. you change the nucleophile to (sodium) bromide and the solvent to DMF 3. you change the leaving group to a tosylate 4. you change the leaving group to NH2 5. you double the concentration of R-X 6. you double the amount of acetone 7. you halved the concentration of acetate 8. you change the starting R-X to (S)-2-bromo-1-methoxy-2-methylhexane (n) Draw a fully labeled reaction energy diagram that is consistent with your chosen type of reaction. Question #2 Explain why the following compound reacts to give a mixture of alkene isomers in which only the Z isomer contains deuterium. Explanation If we changed the stereochemistry at one position in the starting material above, we would generate its _______________________ . What two alkene products would form from this new isomer? What change in reaction conditions could we make to promote more of this style of elimination reaction and avoid any competing substitution? Question #3 (a) When the optically active tosylate (A) was reacted with CH3SNa in DMF, the reaction was observed to be kinetically second order and the substitution product was formed almost exclusively. Show the mechanism and the kinetic rate law for this reaction and predict the steroeochemistry of the product. You may want to follow along with the chart on pages 9 and 10 of SAM. NOTE: tosylate is the leaving group! SCH3 Ph CH3SNa OTs Ph DMF H H3C CH * CH3 A (b) When A was reacted with CH3ONa in CH3OH, the reaction was observed to be kinetically second order. However, the major product became the alkene shown below. Account for this shift in product formation from substitution to elimination via a reaction mechanism. Also, write the kinetic rate law for the elimination and show the stereochemistry of the methyl ether substitution product. You may want to follow along with the chart on pages 9 and 10 of SAM. OCH3 Ph CH3ONa OTs Ph CH3OH H H3 C CH * minor CH3 + Ph major A (c) When A is refluxed in methanol (no added CH3ONa), the SAME substitution and elimination products are formed as in part (b). However, the substitution product now predominates and the stereochemical result is quite different than that observed in part (b). Predict the stereochemistry of the substitution product under these reaction conditions and explain your results by showing a reaction mechanism. Also, write the kinetic rate law for both the substitution and elimination reactions. You may want to follow along with the chart on pages 9 and 10 of SAM. Question #4 When (4Z,3R)-3-bromo-4-methyl-5-phenyl-4-hexene reacts with water (solvent), two regioisomeric substitution products are formed with one product predominating over the other (#2>#1). When small amounts of the hydroxide anion are added to the reaction mixture, no change in rate is observed. H2O (4Z,3R)-3-bromo-4-methyl-5-phenyl-4-hexene 2 regioisomeric products (shown below) Ph OH + ? CH3 regioisomer #1 regioisomer #2 (a) The starting material is named improperly. Provide the correct IUPAC name and provide its structure with correct stereochemistry (draw both a skeletal structure and a Fischer projection)? Correct IUPAC name ______________________________________________________ Fischer skeletal (b) Regioisomer #1 can actually form two different stereochemical products during the course of this reaction. What are the structures corresponding to each of these products and what is the stereochemical relationship between the two? Are they likely to be formed in equal quantities? relationship stereoisomer #1 stereoisomer #2 (c) Provide a complete reaction mechanism that explains how the two different stereoisomers of regioisomer #1 are formed. (d) In part (c), you should have generated a key intermediate structure. This is not the most stable form of this intermediate. SHOW (using the curved arrow formalism) how this intermediate alters itself to achieve a more stable configuration of groups. Resonance is involved, so be sure to draw all of the possible resonance contributors and indicate which is the MOST IMPORTANT. How does this affect the percentage of regioisomers formed (major vs. minor)? Resonance: (e) Given the information provided in part (d), propose a structure for Regioisomer #2. proposed structure (f) Regioisomer #2 produces a new asymmetric carbon. What is the expected stereochemistry for this product? Is it purely R, S, or a combination of the two? Briefly explain your selection. (g) Propose a reaction energy diagram (FULLY LABELED) that shows the energetic course of reaction from reactants to products for the reaction shown above. Question #5 Provide a detailed mechanistic evaluation of the following reactions, being sure that your analysis includes: 1. Accurate structures for each starting material. 2. A summary of your evaluation criteria (substrate structure, solvent effects, leaving group effects, nucleophilicity, basicity, etc.) – see the decision tree in SAM. 3. The correct structures (regiochemistry and stereochemistry) of ALL expected reaction products, including assignment of configuration (R/S or E/Z). 4. An indication of the relative proportions of products (major/minor), if more than one product forms. 5. The complete mechanism of the reactions using structures and the curved arrow formalism. Reaction #1 (S)-2-chloro-2-tert-butylpentane K OCH2CH2CH3 CH3CH2CH2OH Reaction #2 (R)-1-phenyl-2-butanol TsCl CH3CH2OH pyridine Reaction #3 48% HBr (aq) (S)-3-methyl-5-hydroxyl-2-pentanone Question #6 Choices……. (a) Rank the following series of molecules based on reactivity in an SN2 reaction (e.g. NaI in acetone). 1 is fastest, 4 is slowest I Cl Cl Cl (b) Rank the following series of molecules based on reactivity in an SN1 reaction (e.g. EtOH/heat). 1 is fastest, 4 is slowest Br OMe Br Br Br (c) Rank the following series of molecules based on reactivity in an E2 reaction (e.g. NaOEt in EtOH). 1 is fastest, 4 is slowest Me Br Me Br Br Br Me Me (d) Consider the following reaction, (CH3)3CBr H2O (CH3)3COH HBr 1. If the concentration of (CH3)3CBr is doubled, the reaction rate A. doubles B. is halved C. quadruples D. no change 2. If the concentration of H2O is doubled, the reaction rate A. doubles B. is halved C. quadruples D. no change 3. If the concentration of both (CH3)3CBr and H20 are doubled, the reaction rate A. doubles B. is halved C. quadruples D. no change (e) Rank the following series of nucleophiles in methanol. 1 is best, 4 is worst Br NH2 H2O Cl (f) Rank the following series of leaving groups. 1 is best, 4 is worst Br NH2 H2O Cl Question #7 When the compound C5H9Br is reacted with NaOH in DMF, the product that is isolated can be characterized using the following set of spectroscopic data. Using this information come up with the structure of the both the starting material and the product. C5H9Br starting material Product