THE CHEMISTRY OF THE CARBONYL GROUP
advertisement

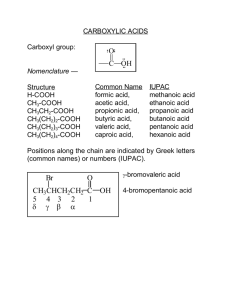
Lecture 6 There are several other reactions of carbonyl compounds which are mechanistically related to the Aldol condensation: H 3C C _ H2C LDA or NaNH2 N Acetonitrile C N _ pKa ca. 25 H2C C N The CN group is somewhat less electron-withdrawing than a carbonyl group - hence the need for a strong base to produce complete deprotonation. O Ph C O _ H 2C C Ph N H - C CH2 C N H H2O Ph HC CH CN -unsaturated nitrile OH OH - - H 2O Ph C CH2 C N H Here the anion of acetonitrile replaces the enolate anion of a basecatalysed aldol condensation. H3C NO2 O _ H2C NaOH N O Nitromethane: pKa ca. 10 _ O The nitro group is extremely electron-withdrawing - hence H2C N O the relatively low pK a of nitromethane and efficient deprotonation by OH - O H2C N O _ O Ph C O _ H2C Ph NO2 H - C CH2 NO2 H H2O Ph HC CH NO2 -unsaturated nitrocompound OH OH Ph - H2O C CH2 NO2 H (nitroalkene) Any functional group that can render a C-H bond acidic enough to result in deprotonation can allow generation of a C-nucleophile which can take part in aldol-like condensations. We will see further examples of such reactions later on in the course. Summary: Aldol and aldol-like condensation reactions: RH2C _ H2C Base Z Z Z = COR; CN, NO2 or other electron-withdrawing group O- O R _ H2C C R Z H C CH2 Z H H2O OH OH _ C CH R Base Z H - H+ R C CH2 Z H - OHR C H C H Z Z = COR; unsaturated carbonyl cpd. Z = CN; unsaturated nitrile Z = NO2; unsaturated nitro-compound i.e. a nitro-alkene * Carboxylic Acids and their Derivatives Streitweiser: Chapters 18 & 19; McMurry (5th Ed.) Chapters 20 & 21. O General formula: R C Z O R O Carboxylic acid C R C OH X Acid halide aka acyl halide O R O C O R Acid anhydride R C Ester OR1 C O O R Amide C NR1 2 An important general reaction for carboxylic acid derivatives (acyl halides, anhydrides, esters and amides) - Addition-Elimination: O R + NuŠ C OŠ Addition R C Z Nu Z O O C C R H R R H-, R- are normally very poor leaving groups. Carbonyl group regenerated. Elimination O R + ZŠ C Nu Carboxylic acids - Synthesis: (1) Oxidation of 1° alcohols: RCH2OH 1 Alcohol KMnO4 - H2 KMnO4 RCHO RCO2H + 'O' Aldehyde Carboxylic acid (B) Hydrolysis of nitriles: O + R C N H or OH R H+ C NH2 Nitrile or OH Amide O R C OH Carboxylic acid (C) Reaction of organomagnesium reagents (aka Grignard reagents) with carbon dioxide (cf. CM2005 lectures): R X Mg R MgX CO2 O R C OMgX H3O+ O R C OH Reactions of carboxylic acids: (1) Acidity O R C O R + H2O C OH O + H3O+ - pKa ca. 4.5 O Resonance-stabilised carboxylate anion: R - O C R C O _ O [Compare HCl (-7); ROH (ca. 16); RCH2COR (ca. 20)] Carboxylate anions are nucleophilic: O R C O- CH3CH2 Br SN2 O R C O Esterification under basic conditions: New bond CH2CH3 Ethyl ester This is not, however, the standard method of making esters which is: H 3O + O R C + HOCH2CH3 OH Esterification under acidic conditions: O R New bond C O CH2CH3 Ethyl ester The mechanism of this reaction will be studied in more detail later. (2) Regiospecific bromination of the alkyl side-chain in alkyl carboxylic acids: O O Br2 C CH3 CH3 C CH3CO2 H CH3 CH2Br -Halogenation of aldehydes and ketones is catalysed by acids and takes place via the small equilibrium concentration of the corresponding enol. CH3 O Br2 O C X C OH BrCH2 OH OH Carboxylic acids do not enolise. C CH2 OH Carboxylic acids do not enolise and hence cannot be -halogenated under similar conditions. CH3CH2 CO2H Trace PBr3 X2 CH3CHXCO2 H -Halogenated carboxylic acid. Phosphorus tribromide catalyses the -halogenation of carboxylic acids. Mechanism: O CH3 CH2 C PBr3 O CH3 CH2 C OH Br Acyl halide O CH3 CH2 C OH CH3 CH C Br Br + OH + OH Acyl halides do enolise. CH3 CH C CH3 Br Br + Br _ CH C Br + Br - Br - H+ O CH3 CH Br C CH3CH2 CO2H O CH3 Br The cycle starts again. CH3 CH C Br OH + O CH2 C Br Esterification of Carboxylic Acids - An Addition/Elimination Reaction H3O+ RCOOR1 + H2O RCOOH + R1 OH O H3C H3O + OH + C H3 C OH C + C H3 C OH OH OH Protonation increases the electrophilicity of the carbonyl carbon atom in the carboxylic acid. + OH H3 C OH C HO OH C C2 H5 H3 C OH + O C2 H5 Tetrahedral intermediate H + OH2 H3C C OH OH H3C C OH +O O C2H5 H C2H5 - H2O + OH H3 C O - H+ C H3 C OEt H2 O C + H3 O+ OEt RCOOH + xs. R1OH H3 O+ RCOOR1 + H2O Ester formation is an equilibrium process - driven towards ester formation by removal of the water co-product and/or use of a large excess of the alcohol. H3O+ RCOOR + xs. H 2O RCOOH + R 1OH The mechanism of ester hydrolysis under acidic conditions simply follows the reverse pathway of the equilibrium steps in the forward process and is driven by an excess of water. Proof that one oxygen atom in the ester is provided by the alcohol is available from labelling experiments: H3O+ O H3C + HO18 C2H5 OH C O H3C C + H2O O18C2H5 The addition-elimination mechanism is central to the reactions of carbonyl compounds derivatives. including carboxylic acids and their Esters can also be synthesised under neutral conditions: O R O C R + CH2N2 OH O CH3 Methyl ester Diazomethane H2C + N _ N _ H2C O R C C + N N O _ OH + H2C R + N C N O + H3C + N Proton transfer O R C O O H3C + N R N C + N2 O CH3 Excellent leaving - group N Hydrolysis of esters and other carboxylic acid derivatives: While esters can be hydrolysed under acidic conditions it is more common to do so under basic conditions - called saponification, 'soap making' (basic ester hydrolysis was a step in early soap manufacture). O O H3C C OH H3C C + OH OC2H5 OC2H5 O O H3C C O + C 2H5OH H3C C OH pKa ca. 16 H3O+ + C 2H5O pKa ca. 4.5 O H3C C OH Other carboxylic acid derivatives are similarly hydrolysed: O O H2O R C R C + NH3 Acid or base OH NH2 O R C Cl O R C O R C O H2O Instantaneous H2O Instantaneous O R C + HCl OH O 2 R C OH