Carboxylic acids
advertisement

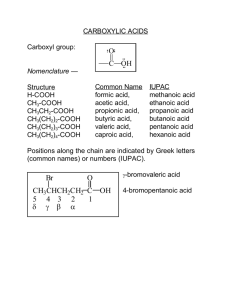
Carboxylic acids: O R-COOH, R-CO2H, R C OH Common names: HCO2H formic acid L. formica ant CH3CO2H acetic acid L. acetum vinegar CH3CH2CO2H propionic acid G. “first salt” CH3CH2CH2CO2H butyric acid L. butyrum butter CH3CH2CH2CH2CO2H valeric acid L. valerans Carboxylic acids, common names: … CH3(CH2)4CO2H caproic acid CH3(CH2)5CO2H --- CH3(CH2)6CO2H caprylic acid CH3(CH2)7CO2H --- CH3(CH2)8CO2H capric acid CH3(CH2)9CO2H --- CH3(CH2)10CO2H lauric acid L. caper goat oil of lauryl 5 4 3 2 1 C—C—C—C—C=O δ γ β α Br CH3CH2CH2CHCOOH bromovaleric acid used in common names CH3 CH3CHCH2COOH -methylbutyric acid isovaleric acid COOH special names benzoic acid COOH CH3 COOH COOH CH3 CH3 o-toluic acid m-toluic acid p-toluic acid IUPAC nomenclature for carboxylic acids: parent chain = longest, continuous carbon chain that contains the carboxyl group alkane, drop –e, add –oic acid HCOOH methanoic acid CH3CO2H ethanoic acid CH3CH2CO2H propanoic acid CH3 CH3CHCOOH 2-methylpropanoic acid Br CH3CH2CHCO2H 2-bromobutanoic acid dicarboxylic acids: HOOC-COOH oxalic acid HO2C-CH2-CO2H malonic acid HO2C-CH2CH2-CO2H succinic acid HO2C-CH2CH2CH2-CO2H glutaric acid HOOC-(CH2)4-COOH adipic acid HOOC-(CH2)5-COOH pimelic acid Oh, my! Such good apple pie! CO2H CO2H CO2H CO2H CO2H CO2H phthalic acid H H C C isophthalic acid terephthalic acid COOH H COOH HOOC maleic acid C C COOH H fumaric acid salts of carboxylic acids: name of cation + name of acid: drop –ic acid, add –ate CH3CO2Na sodium acetate or sodium ethanoate CH3CH2CH2CO2NH4 ammonium butyrate ammonium butanoate (CH3CH2COO)2Mg magnesium propionate magnesium propanoate HO O C OH carbonic acid HO O C ONa sodium bicarbonate sodium hydrogen carbonate NaHCO3 NaO O C ONa sodium carbonate Na2CO3 physical properties: polar + hydrogen bond relatively high mp/bp water insoluble exceptions: four carbons or less acidic turn blue litmus red soluble in 5% NaOH RCO2H + NaOH RCO2-Na+ + H2O stronger acid stronger base weaker base weaker acid RCO2H RCO2- covalent water insoluble ionic water soluble Carboxylic acids are insoluble in water, but soluble in 5% NaOH. 1. Identification. 2. Separation of carboxylic acids from basic/neutral organic compounds. The carboxylic acid can be extracted with aq. NaOH and then regenerated by the addition of strong acid. Carboxylic acids, syntheses: 1. oxidation of primary alcohols RCH2OH + K2Cr2O7 RCOOH 2. oxidation of arenes ArR + KMnO4, heat ArCOOH 3. carbonation of Grignard reagents RMgX + CO2 RCO2MgX + H+ RCOOH 4. hydrolysis of nitriles RCN + H2O, H+, heat RCOOH 1. oxidation of 1o alcohols: CH3CH2CH2CH2-OH + CrO3 CH3CH2CH2CO2H n-butyl alcohol 1-butanol CH3 CH3CHCH2-OH isobutyl alcohol 2-methyl-1-propanol` butyric acid butanoic acid + KMnO4 CH3 CH3CHCOOH isobutyric acid 2-methylpropanoic acid 2. oxidation of arenes: CH3 KMnO4, heat COOH toluene CH3 benzoic acid COOH KMnO4, heat note: aromatic acids only! HOOC H3C terephthalic acid p-xylene KMnO4, heat CH2CH3 ethylbenzene COOH + CO2 benzoic acid 3. carbonation of Grignard reagent: Mg R-X H+ CO2 RMgX RCO2MgX RCOOH Increases the carbon chain by one carbon. CO2 H+ Mg CH3CH2CH2-Br n-propyl bromide RMgX + O C O CH3CH2CH2MgBr O R C O- CH3CH2CH2COOH butyric acid H+ + +MgX O R C OH CH3 CH3 Mg CH3 CO2 Br H+ MgBr COOH p-toluic acid Br2, hv CH3 Mg CH2Br CH2MgBr CO2 H+ CH2 COOH phenylacetic acid 4. Hydrolysis of a nitrile: H2O, H+ R-CN R-CO2H heat H2O, OH- R-CN R-CO2- + H+ R-CO2H heat R-X + NaCN R-CN + H+, H2O, heat RCOOH 1o alkyl halide Adds one more carbon to the chain. R-X must be 1o or CH3! CH3 Br2, hv NaCN CH2Br toluene CH2 CN H2O, H+, heat CH2 COOH phenylacetic acid KCN CH3CH2CH2CH2CH2CH2-Br CH3CH2CH2CH2CH2CH2-CN 1-bromohexane H2O, H+, heat CH3CH2CH2CH2CH2CH2-COOH heptanoic acid CH2OH KMnO4 CH3 KMnO4, heat CO2H Br Mg MgBr C N H2O, H+, heat CO2; then H+ carboxylic acids, reactions: 1. as acids 2. conversion into functional derivatives a) acid chlorides b) esters c) amides 3. reduction 4. alpha-halogenation 5. EAS as acids: a) with active metals RCO2H + Na RCO2-Na+ + H2(g) b) with bases RCO2H + NaOH RCO2-Na+ + H2O c) relative acid strength? CH4 < NH3 < HCCH < ROH < HOH < H2CO3 < RCO2H < HF d) quantitative HA + H2O H3O+ + AKa = [H3O+] [A-] / [HA] ionization in water Ka for carboxylic acids 10-5 Why are carboxylic acids more acidic than alcohols? ROH + H2O H3O+ + RORCOOH + H2O H3O+ + RCOOΔGo = -2.303 R T log Keq The position of the equilibrium is determined by the free energy change, ΔGo. ΔGo = ΔH - TΔS ΔGo ΔH Ka is inversely related to ΔH, the potential energy difference between the acid and its conjugate base. The smaller the ΔH, the larger the Ka and the stronger the acid. potential energy H3O+ + A- ΔH HA + H2O ionization The smaller the ΔH, the more the equilibrium lies to the right, giving a larger Ka ( a stronger acid ). OR C O O R C O- O R C O Resonance stabilization of the carboxylate ion decreases the ΔH, shifts the ionization in water to the right, increases the Ka, and results in carboxylic acids being stronger acids. Effect of substituent groups on acid strength? CH3COOH 1.75 x 10-5 ClCH2COOH 136 x 10-5 Cl2CHCOOH 5,530 x 10-5 Cl3CCOOH 23,200 x 10-5 -Cl is electron withdrawing and delocalizes the negative charge on the carboxylate ion, lowering the PE, decreasing the ΔH, shifting the ionization to the right and increasing acid strength. Effect of substituent groups on acid strength of benzoic acids? Electron withdrawing groups will stabilize the anion, decrease the ΔH, shift the ionization to the right, increasing the Ka, increasing acid strength. COOG Electron donating groups will destabilize the anion, increase the ΔH, shift the ionization in water to the left, decreasing the Ka, decreasing acid strength. COOG -NH2, -NHR, -NR2 -OH -OR -NHCOCH3 -C6H5 -R -H -X -CHO, -COR -SO3H -COOH, -COOR -CN -NR3+ -NO2 electron donating electron withdrawing Relative acid strength? Ka p-aminobenzoic acid 1.4 x 10-5 p-hydroxybenzoic acid 2.6 x 10-5 p-methoxybenzoic acid 3.3 x 10-5 p-toluic acid 4.2 x 10-5 benzoic acid 6.3 x 10-5 p-chlorobenzoic acid 10.3 x 10-5 p-nitrobenzoic acid 36 x 10-5 2. Conversion into functional derivatives: a) acid chlorides O R C OH SOCl2 O R C Cl or PCl3 orPCl5 CO2H + SOCl2 O CH3CH2CH2 C OH COCl PCl3 O CH3CH2CH2 C Cl b) esters “direct” esterification: H+ RCOOH + R´OH RCO2R´ + H2O -reversible and often does not favor the ester -use an excess of the alcohol or acid to shift equilibrium -or remove the products to shift equilibrium to completion “indirect” esterification: RCOOH + PCl3 RCOCl + R´OH RCO2R´ -convert the acid into the acid chloride first; not reversible O C OH H+ + CH3OH SOCl2 O C Cl CH3OH O + H2O C O CH3 c) amides “indirect” only! RCOOH + SOCl2 RCOCl + NH3 RCONH2 amide O PCl3 OH 3-Methylbutanoic acid O NH3 Cl O NH2 Directly reacting ammonia with a carboxylic acid results in an ammonium salt: RCOOH + NH3 RCOO-NH4+ acid base O C OH PCl3 O C Cl NH3 O C NH2 amide NH3 O C O NH4 ammonium salt 3. Reduction: RCO2H + LiAlH4; then H+ RCH2OH 1o alcohol LiAlH4 H+ CH3CH2CH2CH2CH2CH2CH2COOH Octanoic acid (Caprylic acid) CH3CH2CH2CH2CH2CH2CH2CH2OH 1-Octanol Carboxylic acids resist catalytic reduction under normal conditions. RCOOH + H2, Ni NR O CH2 C OH H2, Pt LiAlH4 H+ CH2CH2OH NR 4. Alpha-halogenation: (Hell-Volhard-Zelinsky reaction) RCH2COOH + X2, P RCHCOOH + HX X α-haloacid X2 = Cl2, Br2 CH3CH2CH2CH2COOH + Br2,P pentanoic acid COOH Br2,P NR (no alpha H) CH3CH2CH2CHCOOH Br 2-bromopentanoic acid RCH2COOH + Br2,P RCHCOOH + HBr + H; O Na H n the Br NH3 RCHCOOH RCHCOOH NH2 OH KOH(alc) RCH2CHCOOH Br then H+ RCH=CHCOOH aminoacid 5. EAS: (-COOH is deactivating and meta- directing) CO2H HNO3,H2SO4 NO2 CO2H CO2H H2SO4,SO3 SO3H CO2H benzoic acid Br2,Fe Br CH3Cl,AlCl3 NR spectroscopy: IR: -COOH O—H stretch 2500 – 3000 cm-1 (b) C=O stretch nmr: -COOH 10.5 – 12 ppm 1680 – 1725 (s) p-toluic acid -COO—H stretch C=O COOH c b CH3 c a b a Carboxylic acids, syntheses: 1. oxidation of primary alcohols RCH2OH + K2Cr2O7 RCOOH 2. oxidation of arenes ArR + KMnO4, heat ArCOOH 3. carbonation of Grignard reagents RMgX + CO2 RCO2MgX + H+ RCOOH 4. hydrolysis of nitriles RCN + H2O, H+, heat RCOOH carboxylic acids, reactions: 1. as acids 2. conversion into functional derivatives a) acid chlorides b) esters c) amides 3. reduction 4. alpha-halogenation 5. EAS