Carboxylic acids
Dr AKM Shafiqul Islam
School of Bioprocess Engineering
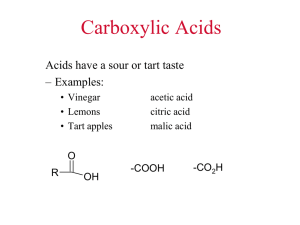
Carboxylic Acids
The functional group of a carboxylic acid is a
carboxyl group,
Carbonyl with hydroxy
represented in one of three ways
O
C-OH
COOH
CO2 H
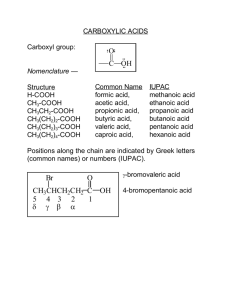
Fill in the table below.
Compound
Common Name
IUPAC Name
HCOOH
formic acid
methanoic acid
CH3COOH
acetic acid
ethanoic acid
CH3CH2COOH
propionic acid
propanoic acid
CH3(CH2)2COOH
butyric acid
butanoic acid
CH3(CH2)3COOH
pentanoic acid
CH3(CH2)4COOH
valeric acid
caproic acid
C6H5COOH
benzoic acid
benzoic acid
hexanoic acid
2. Carboxylic acids that have branches are often
given IUPAC names, where the numbering of the
longest carbon chain begins at the Carbonyl
carbon. Name the following compounds.
Compound
Br
O
CH3 CH
C
Cl
CH3 CH
2-bromopropanoic acid
OH
O
CH2 C
NH2 CH3
CH3 CH2 CH
IUPAC Name
CH
3-chlorobutanoic acid
OH
O
CH2 C
OH
4-amino-3-methylhexanoic acid
Carboxylic Aromatic Acids
HO
HO
O
C
C
HO
O
O
C
OH
OH
C
O
salicylic acid
o-phthalic acid
(2-hydroxybenzenecarboxylic)
(benzene-1,2-dicarboxylic)
benzoic acid
HO
O
HO
C
O
C
CH
CH
HO
gallic acid
(3,4,5-trihydroxy
-benzenecarboxylic)
C
CH3
OH
OH
O
O
OH
vanillic acid
(4-hydroxy-3-methoxy
-benzenecarboxylic)
OH
cinnamic acid
{(2E)-3-phenylprop
-2-enoic}
Where are they to be found?
Carboxylic acids and their derivatives:
Tart taste of citrus fruits, vinegar, and rhubarb
Sharp sting of red ants
Unsavory smell and taste of rancid butter
Vitamin C is a carboxylic acid
Pleasant taste and odor of fruits are due to carboxylic acid
derivatives: esters
Physical Properties
The carboxyl group contains three polar
covalent bonds; C=O, C-O, and O-H
the polarity of these bonds determines the major
physical properties of carboxylic acids
δ-O
– Hδ+
δ+C=Oδ-
R
Electron Delocalization
R
C
•• •
O•
•• O ••
R
+
C
•• •–
O•
••
•• O ••
H
H
Electron Delocalization
R
C
•• •
O•
R
•• O ••
+
C
•• •–
O•
••
•• O ••
H
C
+ O ••
H
R
•• •–
O•
••
stabilizes carbonyl group
H
Physical Properties
The carbonyl group has a large dipole
The hydroxy group is capable of hydrogen bonding.
The molecules can H-bond to each other
How does this affect boiling point?
Higher than aldehydes and ketone – no H-bonds
Higher than alcohols – H-bonds, not strong dipole
hydrogen bondin g
betw een tw o
molecules
H3 C
O
+
H
O
C
C
O
H
+
O
-
CH3
Solubility in Water
Carboxylic acids are similar to alcohols in respect
to their solubility in water
Form hydrogen bonds to water
H
O
H
O
H3CC
H
O
H
O
H
Physical Properties
carboxylic acids are more soluble in water than are
alcohols, ethers, aldehydes, and ketones of comparable
molecular weight
Structu re
N ame
CH3 COOH
CH3 CH2 CH2 OH
CH3 CH2 CHO
acetic acid
1-prop anol
prop anal
Boilin g
Solubility
Molecular Poin t
Weigh t
(°C) (g/100 mL H 2O)
60.5
118
infinite
97
60.1
infinite
58.1
48
16
CH3 (CH2 ) 2 COOH butan oic acid
CH3 (CH2 ) 3 CH2 OH 1-pentan ol
pentan al
CH3 (CH2 ) 3 CHO
88.1
88.1
86.1
163
137
103
infinite
2.3
slight
R OH
acid
+ H2O
base
R
O
+
+
H3O
alkoxide anion
Alkoxide anions don’t have resonance-stabilization.
Alcohols are weaker acids than carboxylic acids.
Copyright© 2005, Michael J. Wovkulich. All rights reserved.
Physical Properties
Sharp and or sour odor/taste
Vinegar, rancid butter, sweat, sauerkraut
Carboxylic acids derivatives
When the carbonyl group is substituted by atoms other
than carbon or hydrogen, as in
O
R
C
Z
where Z is oxygen, nitrogen, or halogen, the compound
becomes a carboxylic acid or a carboxylic acid
derivative.
Carboxylic acid and derivatives
Ester
Acid anhydride
Amide
R
R
O
R
R'O
O
R
O
O
Acid halide
R
O
H2 N
O
X
Carboxylic Acid Derivatives
Hydroxy Acids
Beta-hydroxy acid
Salicylic Acid
exfoliant
oil soluble – penetrates oil containing pores to remove
dead skin cells
less irritating than alpha acids
O
OH
OH
Reactions carboxylic acids
1.
2.
as acids
conversion into functional derivatives
a)
b)
c)
3.
4.
5.
acid chlorides
esters
amides
reduction
alpha-halogenation
EAS
Reactions : Acid/Base
Carboxylic acids are weak acids
Give up the H bonded to O to water or base
Tendency for acid to give up proton (H+) is indicated by
pKa; lower pKa indicates stronger acid
Carboxylic acids: pKa 4.0 - 5.0
O
CH3COH
O
H2O
In comparison:
Hydrochloric acid
Sulfuric acid
Alcohol
CH3CO
H3O+
pKa = -7
pKa = -3
pKa = 15-16
Comparison Of pKa Values
Hydrochloric acid
Sulfuric acid
Alcohol
Carboxylic acid
pKa = -7
pKa = -3
pKa = 15-16
pKa = 4
Acidity of Carboxylic Acids
1. Both acetic acid and ethanol have an acidic
hydrogen on the OH group. Why is the pKa value for
acetic acid (pKa = 4.74) lower than the pKa for
ethanol (pKa = 15.9)? In other words, why are
carboxylic acids more acidic than alcohols. Consider
resonance-stabilized anions.
O
O
R
C
acid
OH + H2O
base
R
C
O
carboxylate
anion
+
+
H3O
O
O
R
R
C
=
C
O
O
O
R
C
O
½½-
resonance-stabilized carboxylate anion
O
O
R
C
acid
OH + H2O
base
R
C
O
+
+
H3O
carboxylate
anion
The greater the stability of the carboxylate anion, the farther
the shift to the right.
The farther the shift to the right, the greater the acidity.
As acids:
a) with active metals
RCO2H + Na RCO2-Na+ + H2(g)
b) with bases
RCO2H + NaOH RCO2-Na+ + H2O
c) relative acid strength?
CH4 < NH3 < HCCH < ROH < HOH < H2CO3 < RCO2H < HF
d) quantitative
HA + H2O H3O+ + A- onization in water
Ka = [H3O+] [A-] / [HA]
Dehydration of Carboxylic Acid
2 H3C COOH
O
800o
O
+ H2O(g)
H3C
O
CH3
O
COOH
200o
O
+ H2O(g)
COOH
O
* water must be removed (to avoid hydrolysis)
* limited use
Reaction With Bases
All carboxylic acids react with strong bases to form
water-soluble salts
COOH + NaOH
H2 O
Ben zoic acid
(slightly soluble in w ater)
COOH +
+
COO Na + H2 O
Sodiu m b enzoate
(60 g/100 mL w ater)
NH3
Benzoic acid
(s ligh tly solub le in w ater)
H2 O
-
COO NH4
+
Ammoniu m b enzoate
(20 g/100 mL water)
Reactions with bases
Salts of carboxylic acids
Drop the –ic acid
Change to –ate
Sodium benzoate & monosodium glutamate
Sodium benzoate – inhibit mold
MSG – flavor enhancer
O
O
O
-
C
+
O Na
HO
+
CH2
C
- Na
CH2
O
CH
NH2
sodium benzoate
MSG
Conversion into functional derivatives
a) acid chlorides
O
R C
OH
SOCl2
O
R C
Cl
or PCl3
orPCl5
CO2H + SOCl2
O
CH3CH2CH2 C
OH
COCl
PCl3
O
CH3CH2CH2 C
Cl
b) esters
“direct” esterification:
H+
RCOOH + R´OH RCO2R´ + H2O
-reversible and often does not favor the ester
-use an excess of the alcohol or acid to shift
equilibrium
-or remove the products to shift equilibrium to
completion
“indirect” esterification:
RCOOH + PCl3 RCOCl + R´OH RCO2R´
-convert the acid into the acid chloride first; not
reversible
O
C
OH
H+
+
CH3OH
SOCl2
O
C
Cl
CH3OH
O
+ H2O
C
O CH3
c) amides
“indirect” only!
RCOOH + SOCl2 RCOCl + NH3 RCONH2
amide
O
OH
3-Methylbutanoic acid
PCl3
O
NH3
Cl
O
NH2
Directly reacting ammonia with a carboxylic acid results in an
ammonium salt:
RCOOH + NH3 RCOO-NH4+
acid
base
O
C
OH
PCl3
O
C
Cl
NH3
O
C
NH2
amide
NH3
O
C
O
NH4
ammonium salt
3. Reduction:
RCO2H + LiAlH4; then H+ RCH2OH
1o alcohol
LiAlH4
H+
CH3CH2CH2CH2CH2CH2CH2COOH
Octanoic acid
(Caprylic acid)
CH3CH2CH2CH2CH2CH2CH2CH2OH
1-Octanol
Carboxylic acids resist catalytic reduction under normal
conditions.
RCOOH + H2, Ni NR
O
CH2 C
OH
H2, Pt
LiAlH4
H+
CH2CH2OH
NR
4. Alpha-halogenation: (Hell-Volhard-Zelinsky
reaction)
RCH2COOH + X2, P RCHCOOH + HX
X
α-haloacid
X2 = Cl2, Br2
CH3CH2CH2CH2COOH
+
Br2,P
pentanoic acid
COOH
Br2,P
NR (no alpha H)
CH3CH2CH2CHCOOH
Br
2-bromopentanoic acid
RCH2COOH + Br2,P
RCHCOOH + HBr
+
H;
O
Na
H
n
the
Br
NH3
RCHCOOH
RCHCOOH
NH2
OH
KOH(alc)
RCH2CHCOOH
Br
then
H+
RCH=CHCOOH
aminoacid
5. EAS: (-COOH is deactivating and meta- directing)
CO2H
HNO3,H2SO4
NO2
CO2H
CO2H
H2SO4,SO3
SO3H
CO2H
benzoic acid
Br2,Fe
Br
CH3Cl,AlCl3
NR