Electron Orbits Binding Energy
advertisement
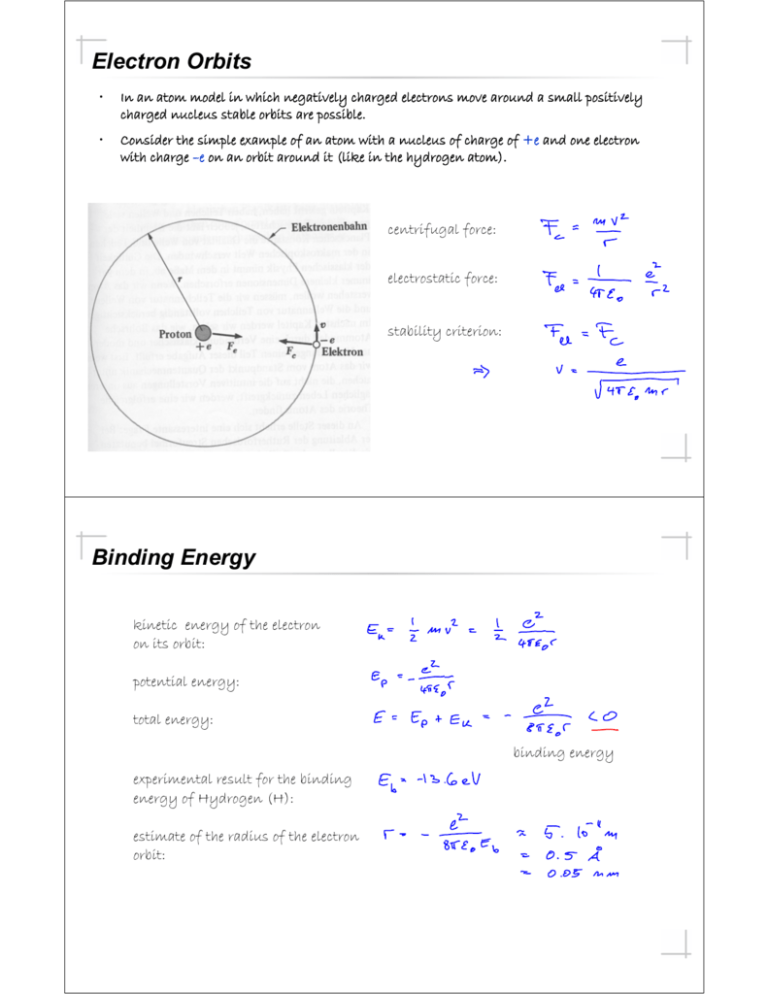
Electron Orbits • In an atom model in which negatively charged electrons move around a small positively charged nucleus stable orbits are possible. • Consider the simple example of an atom with a nucleus of charge of +e and one electron with charge –e on an orbit around it (like in the hydrogen atom). centrifugal force: electrostatic force: stability criterion: Binding Energy kinetic energy of the electron on its orbit: potential energy: total energy: binding energy experimental result for the binding energy of Hydrogen (H): estimate of the radius of the electron orbit: Radiation of Electron on Orbit electromagnetic power radiated by an charge moving with acceleration : centrifugal acceleration of electron on circular orbit: power radiated by electron in a hydrogen atom (r ~ 0.05 nm): • Under classical considerations this electron should loose its energy (-13,6 eV) very rapidly and drop into the nucleus (which would be very bad news). • Considering the wave-like properties of the electron however we will be able to explain (using the laws of quantum mechanics) why the electron moves on a stable orbit around the nucleus. Emission and Absorption of Light measuring the spectrum of light Black Body Spectrum • fusion (H2 -> He) • power ~ 100. 109 GW • temperature T ~ 6000 Kelvin • continuous spectrum • power on earth 1 kW/m2 • largest intensity in visible part of the spectrum Spectrum of the Sun Spectra Na () visible spectrum of hydrogen Sodium Doublet • transition between two electronic states with different orbital angular momenta L • evidence for electron spin due to spin-orbit coupling visible in total angular momentum j • naming convention of levels: n Lj More Spectra • the sun • sodium (Na) • mercury (Hg) • lithium (Li) • hydrogen (H) Emission Spectra • hot and dense objects (solids) display continuous spectra (e.g. the sun) • the properties of a large number of interacting atoms (collisions) is observed in this case • frequently the spectrum can be explained using the ideas of black body radiation (to be discussed later) • the properties of individual atoms become apparent at low densities, when the interactions are small • then individual line spectra are observed • the properties of these spectra are characteristic for the different elements • both emission and absorption spectra can be observed Spectral Series • in the late 19th century it was found that spectral lines of simple elements can be ordered into simple series • one of the first ones was the Balmer (n=2) series of spectral lines in Hydrogen in the visible wave lengths. n=5 n=4 n=3 n=2 Balmer series n=1 Bohr Model • If the electron orbit length would not be an integer multiple destructive interference would appear and the orbit would not be stable or even exist. Quantum Numbers in the Bohr Model Energy Levels of the Hydrogen Atom total energy of electron as calculated before with nth Bohr radius: with Rydberg constant The set of different energies are the of the is the energy hydrogen atom. . are corresponding to the quantum number energies with the corresponding quantum numbers. Spectral Lines and Transitions in Hydrogen Excitation, Relaxation and Lifetime atoms are excited into higher energy states • by collisions with electrons or other atoms(Franck-Hertz experiment) • by absorption of photons (c.f. absorption spectra) after some time the atoms relax (decay) back to the ground state the life time of an excited state • is limited by spontaneous emission (coupling to vacuum fluctuations, Einstein A coefficient to be discussed later) • the emission can also be induced by interactions with other photons, collisions with electrons or other atoms • a wide range of life times is observed, a few nanoseconds to minutes (if you are very careful) Experiment of Franck and Hertz vacuum tube with mercury (Hg) vapor experimental set up • original measurement result The experiment demonstrates that atoms absorb energy from collisions with electrons in quanta that are determined by the atoms energy level structure given by the laws of quantum mechanics. Nobel Prize in Physics (1925): Franck and Hertz "for their discovery of the laws governing the impact of an electron upon an atom" James Franck 1/2 of the prize Gustav Ludwig Hertz 1/2 of the prize Germany Germany Goettingen University Goettingen, Germany Halle University Halle, Germany b. 1882 d. 1964 b. 1887 d. 1975 Experimental Setup Spectrum of Neon • excitation by electron collision • highly excited electronic states • • transitions in the visible frequency range lower excited electronic states ground state Comment of Franck on their Experiment "It might interest you to know that when we made the experiments that we did not know Bohr's theory. We had neither read nor heard about it. We had not read it because we were negligent to read the literature well enough -- and you know how that happens. On the other hand, one would think that other people would have told us about it. For instance, we had a colloquium at that time in Berlin at which all the important papers were discussed. Nobody discussed Bohr's theory. Why not? The reasons is that fifty years ago, one was so convinced that nobody would, with the state of knowledge we had at that time, understand spectral line emission, so that if somebody published a paper about it, one assumed, "Probably it is not right." So we did not know it. But we made that experiment (and got the result that confirmed Bohr's theory) because we hoped that if we found out where the borderline between elastic and inelastic impact lies ... only one line might appear. But we did not know whether that would be so, and we did not know whether at all an emission of an atom is of such a type that one line alone can be emitted and all the energy can be used for that purpose. The experiment gave it to us, and we were surprised about it. But we were not surprised after we read Bohr's paper later, after our publication." -- Excerpt from one of three recordings of J. Franck, made in connection with a film on the Franck-Hertz experiment at Educational Services, Inc., Watertown, Massachusetts, in January, 1961. As transcribed in "On the recent past of physics", by Gerald Holton, American Journal of Physics, vol. 29, p. 805 (1961). Correspondence Principle • The prediction of quantum theories, like the (maybe too) simple Bohr model, should correspond to the predictions of classical theories in the limit of large quantum numbers. This fact is called the correspondence principle. Hydrogen-Like Atoms extension of Bohr model to other atoms with a single electron He+, Element(Z-1)+ energy levels in potential scaled with atomic number








