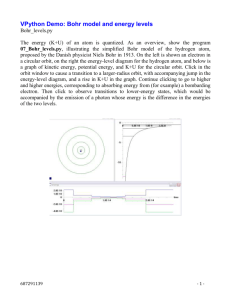
Note: The Bohr Model of the Hydrogen Atom
advertisement

CH101 Notes on the Bohr Model of the Hydrogen Atom
Neils Bohr was the first to attempt a fusion of classical mechanics and quantum theory to explain
the nuclear model of the hydrogen atom and its line spectra. His assumptions were as follows.
1. The electron moves in a circular path around the nucleus.
2. The energy of the electron can take on only certain quantized values.
3. The only allowed orbits (stable orbits) are those where the angular momentum is an integral
multiple of ħ: meru = nħ (1)
(n = 1, 2, 3,.. ) {ħ = h/2)
4. The electron can only absorb or emit electromagnetic radiation (h) when it moves from one
stable orbit to another.
Starting by considering the electron in circular motion around the proton, equation 2 was arrived
at as shown below.
The total energy of the electron (= V + K) was then deduced:
Imposition of the quantum restriction meru = nħ (1) on angular momentum gives equation 4 (by
combination of equations 1 and 2)
Substitution for u in equation 3 gives the Bohr Equation for quantized energy levels and
equations 1 and 4 can be used to derive the expression for the radii of the Bohr orbits.
The integers n, introduced with the quantum hypothesis, define the energy for each stable orbit:
the energy level. Each energy level has its own, unique value of n, called the first quantum
number. The higher the value of n, the higher the algebraic value of the energy level. When an
electron shifts from a level with a high quantum number to one with a lower quantum number,
radiation is emitted. Electron shift occurs in the opposite direction when radiation is absorbed.
Thus, the Bohr model can explain and predict the wavelength of all the lines in each of the
emission spectra (Lyman series, Balmer series, etc) of hydrogen or hydrogen-like ions.
n3
n2
n1
Photon
n3