GroupWk13Soln
advertisement

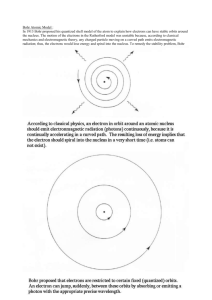
Physics 208, Spring 08, Group Problem Week 13 Problem on the Bohr model of the Hydrogen atom In the Bohr’s model the H-atom has a proton in the nucleus and an electron orbiting in circular allowed orbits. Remember that: Planck’s constant: h = 6.626 x 10-34 m2 kg/s Reduced Planck constant: ℏ = h/2π= 1.055 x 10-34 J s. Planck’s constant x velocity of light hc = 1240 eV x nm electron mass: 9.11 x 10-31 kg, melectron c2 = 511,000 eV A) Calculate the velocity and the radius of the electron in the first allowed orbit from the fact that you know that there is an electric force between the nucleus and the electron and the quantization condition on the angular momentum of the electron postulated by Bohr. For each of the results check the dimensions of your result. Solution: e2 v2 e2 F k 2 m a0 k (1) a0 a0 mv 2 On an orbit n the quantization condition of the angular momentum says: L = mvr = pr= nℏ hence for the first orbit v= ℏ/(ma0) (2). Inserting (1) in (2): v ma 0 mv 2 ke2 9 10 9 (1.6 1019 ) 2 v 2.18 10 6 m /s 2 34 mke 1.055 10 The radius of the first orbit (also called Bohr radius) is: 1.055 1034 a0 0.0.053nm mv 9.11031 kg 2.18 10 6 B) Calculate the total energy of the electron in the first orbit.This is the energy that needs to be provided to the electron in the first energy level to ionize the atom. Solution: p2 e2 E k 2m a0 e2 e2m 2 2 F k mv p k a0 a0 Ek e 2m e2 e2 9 10 9 (1.6 1019 ) 2 k k 2.17 1018 J 13.6eV 9 a0 2m a0 2a0 2 0.053 10 C) Calculate the wavelength of the light emitted as a hydrogen atom undergoes a transition from state n = 4 to n = 2. To which series of the H-atom emission spectrum does this line belong and in what range of the EM spectrum is it? Solution: The energy of the energy levels of the H-atoms are En = -13.6eV/n2. Hence 13.6 13.6 hc hc E4 E2 2.55eV 1240nmeV /2.55eV 486nm 16 4 E Balmer, Blue in the visible